Bradford Assay: Establishing a Standard Curve for Protein
advertisement

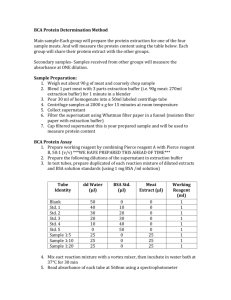
Module 3. Establishing Standard Curve (Bradford Assay) 1. Melissa Myoglobin and the Missing Molecules Melissa Myoglobin had a little tube filled with a clear protein solution. To do more research with this protein, she needed to know some of its properties, including concentration. She knew that to determine the concentration, she needed to know two things: the amount of protein and the volume of the solution (for example, moles/L). She used a micropipette to determine the volume but what about the amount? Could she look at the solution under a microscope and count the molecules? No, even with a microscope, she could not see the protein molecules because they are so small. If she cannot count the molecules, how does she determine the amount in the tube? Think back to Module 2: You measured something that was proportional to NADPH concentration: the absorbance of light. This would work well for Melissa except for the fact that unlike NADPH, the protein does not absorb enough light for a practical measurement at typical concentrations. Melissa had to use a reagent that produces a colored compound when in presence of the protein. The absorbance of the solution is proportional to concentration: the more protein molecules in the tube, the more colored compounds are produced, and the higher the absorbance of the solution. Melissa added the Coomassie reagent to the protein sample and measured the absorbance value to be 0.7. What is the concentration? An absorbance value alone tells Melissa very little about the concentration. She needs a standard for comparison. What is a standard? Melissa thought about the time when an alien from a foreign planet visited the classroom. The alien was sad because he did not know how tall he was and all the kids in the class new their heights. The alien was taller than the shortest kid and shorter than the tallest student. The class decided all they needed was a measuring stick but their teacher, Dr. Scatterbrainius, could not find one. So the students decided to create a number of standards that span the full range of possible heights. They lined up from the shortest to the tallest and had the alien find the two kids he was the closest in height to. The alien then asked these two for their heights, and voila, the alien knew his own height by comparison. The picture on a roll of pH paper is another example of a standard for comparison; here you observe the color of the paper to determine pH of a solution (see figure). The color of the paper is a “proxy” for the pH of the solution. Children are often mesmerized by how scientists “magically” collect data and information. How do they know where and what to look for and where does all that information come from? One step in growing into a scientist happens when you realize how much of data is acquired by “comparing”. Machines and techniques rely on comparisons, for example, between an unknown and known concentration of a compound. In order to give a number to “knowledge”, there must often be a scale or a set of standards. In this lab, you can learn to set up “a scale” for measuring protein concentrations. 2. Purpose of the lab The instructor will provide you with solution of the protein Bovine Serum Albumin (BSA) with a concentration of 2 mg/mL (attention: the concentration units are mass per volume). Your assignment is to follow a protocol to establish the standard curve for BSA that shows the relationship between absorbance and protein concentration. You will make solutions with different known BSA concentrations, add each to a colorimetric reagent, and measure the resulting absorbance values. You will make a graph that has absorbance on the y-axis and protein concentration on the xaxis. This is called a standard curve, a kind of a ruler that tells you what absorbance values to expect for specific protein concentrations. In other words, you will determine the relationship between absorbance and protein concentration. 1 3. Agenda for the Day • • • • • 4. Background • • • • 5. You must have worked through Math Moment problems before coming to class. Upon entry, each student shows their work to instructor to receive points. Presentation on preparing a standard curve, its limits, and associated challenges. In class, groups review Math Moment Problems. Conduct the experiment in groups. Each student individually records data in their personal laboratory notebook. Clean up Useful information can be found Chapter 3A under section” Buffer dilutions” and 3B under section “The Bradford Assay” in the textbook (Boyer). Information about the assay can be found in the following link: http://www.bio-rad.com/webroot/web/pdf/lsr/literature/LIT33.pdf In Bradford Assay, a reagent molecule (Coomasie Brilliant Blue) binds to a protein and changes the color of the solution. A spectrophotometer can be used to read the absorbance at the appropriate the wavelength. The absorbance values increase with increasing protein concentrations. Math Moment 1. Dilution calculation, you can use C1V1 = C2V2. How many milliliters of water should be added to 15.0 mL of 0.50 M Tris buffer to make 0.15 M Tris buffer? Hint: The final volume (V2) is the sum of the volume of the water and the volume of the Tris buffer (V1). 2. Fill in the blanks in the linear dilution table shown below. Assume that the stock solution is 2.0 M and you require 20 mL diluted samples of the following final concentrations: 1.66 M, 1.33 M, 1.0 M, 0.66 M, 0.33 M, and 0.00 M. Stock (M) 2.0 2.0 2.0 2.0 2.0 2.0 Final Dilution (M) 1.66 1.33 1.0 0.66 0.33 0.00 Stock (mL) Water (mL) Final Volume (mL) 20 20 20 20 20 20 Table for a linear dilution process. Fill in blanks. A dilution is the process of combining specific volumes of a solution of analyte with solvent to make less concentrated solutions of analyte. This is one of the important skills that you need to master during your undergraduate training because it is done again and again as part of biochemical research. What mass of protein is present in a sample that has an Absorbance of 0.3 based on the standard curve shown? 0.6 Absorbance 3. Given the protein standard curve in the figure, is the X-axis shown in units of mass or concentration? ________________ 0.4 0.2 0 0 10 20 30 40 50 60 70 80 90 microgram protein 100 110 120 130 2 4. If 10 uL of protein sample was added to the assay in the question above, what was the concentration of the protein sample in units of ug/uL? Hint: From the standard curve above, you know the mass in ug. You know the volume of the sample used on the assay in uL. By divinding mass by volume (ug/uL), you will be able to determine the concentration. 5. What is the concentration in the question above in units of mg/mL? Hint: This is a unit conversion from ug/uL to mg/mL. 6. In the standard curve above, please, convert the values on the xaxis from mass (ug) to concentration (ug/uL) of protein sample. Note: Concentration of protein sample refers to the sample that was made before adding anything to the wells. The volume of protein used in the assay is 10 uL for each data point. What are the concentrations on the x-axis that correspond to 20 ug, 40 ug, 60 ug, 80 ug, and 120 ug (Fill in table)? For this question, you will simply need to divide the amount of protein (mass in ug) by volume in uL. Notice that you can express the standard curve as absorbance vs. protein concentration or absorbance vs. protein amount. Mass of protein in assay (ug) 20 40 60 80 120 Concentration of protein in assay (ug/uL) 7.a. A student added 200 uL of Coomassie Reagent and 10 uL of protein sample (0.1 mg/mL) into a well in a microtiter plate. As stated above, the concentration of the protein sample in question is 0.1 mg/mL. What is the concentration (mg/mL) of the protein in the well (once mixed with the Coomassie Reagent)? 7.b. What is the amount of protein in the well in units of ug? 8. Solve the following equations for x. y = mx + b y= 6. Supplies Provided • • • • • • • • • Ice buckets and ice BSA protein standard at a concentration of 2.0 mg/mL - KEEP IT ON ICE! Buffer Bradford Reagent (already diluted to 1X) Eppendorf tubes Micropipettors and tips (multi-channel pipettors) 96-well microtiter plates Plate shaker U-VIS microtiter plate spectrophotometer 3 7. Protocol Prepare dilutions of the 0.2 mg/ml BSA standard (BSA, a commonly used reference protein). Use a serial dilution approach in which you dilute the 0.2 mg/ml BSA standard two-fold for a total of seven times: 1. 2. 3. 4. 5. 6. 7. Label 7 eppendorf tubes with #1 through 7. Add 75 ul water to tubes #2-7, and 200 ul BSA standard to tube #1. Transfer 75 ul of BSA standard from tube #1 to tube #2, mix by flicking the tube. Tube #2 now contains 2x diluted BSA standard. Transfer 75 ul of this 2x diluted BSA standard from tube #2 to tube #3. Tube #3 now contains 4x diluted BSA standard. Continue this dilution series to tube #7. Next, assemble your standard samples into a microtiter plate as follows. Standard samples are assayed in triplicate, at a volume of 20 ul. Transfer 20 ul of each standard sample into the plate according to the following plate map. START TRANSFERING THE LOWEST CONCENTRATION SAMPLES AND WORK YOUR WAY UP – THIS MEANS YOU DO NOT NEED TO CHANGE PIPET TIPS. Wells H1-3 represent the blank or background wells, where only water is added. 8. 1 5 6 7 8 9 10 11 12 A STD 1 STD 1 STD 1 B STD 2 STD 2 STD 2 C STD 3 STD 3 STD 3 D STD 4 STD 4 STD 4 E STD 5 STD 5 STD 5 F STD 6 STD 6 STD 6 G STD 7 STD 7 STD 7 9. 10. 11. 12. 13. 14. 2 3 4 Add 150.0 µL of the Coomassie Reagent supplied to you by the instructor to each well. Snap plate onto the plate shaker, mix for 30 seconds. Be careful to not mix too fast so that the solutions leave the wells Incubate plate for 10 minutes at room temperature without shaking. Measure the absorbance of the samples in the plate at 564 nm using the plate reader. Note, do not push the tray or press down on it. It will go in automatically and you will break the platereader if you manually push it in. Note, only one of the absorbance plate readers in the lab has the appropriate filter for 564 nm and each group must use this instrument Once all measurements have been done, use a black marker to mark the bottom of the wells that have been used on your plate and save the plate for future use. Prepare standard curve as follows: o Calculate the average absorbance of each of your duplicates. o Plot absorbance values of standards on y-axis and µg/ml BSA** on x-axis (**this is the concentration before mixing the sample with the Bradford reagent). 4 8. Common Mistakes and Some Advice • • • • • • • • • • • 9. There will be much pipetting. Remember good pipetting technique. Work in an orderly manner, record all your work, and be as accurate as possible. Before using a pipettor, double-check that the volume you are wishing to aspirate is correct (and not tenfold bigger or smaller). Please, handle pipettors gently. When adding the reagent to the wells, do not empty the pipettor to the second stop (that makes lots of bubbles) and do have the tip touch the sidewall of the well. Keep your BSA solutions on ice to prevent denaturation. After the 96-well is ready, place it briefly on the plate shaker. The plate “snaps” onto the holder in the shaker. Do this gently. The plate should be incubated at least 5 minutes before placing it in the reader. You will measure the absorbance of the samples in the plate using a plate reader. Note: after gently snapping the plate onto the plate holder, do not push the tray in. You will control the plate reader from the computer. The plate will go in automatically and you will break the plate-reader if you manually push the plate in. The mechanism is very delicate. You can use the equation C1V1=C2V2 to determine the BSA concentrations in tubes 1-7. Once you have the absorbance values, take a picture of the computer screen and then type the values in a data sheet in excel. Plot the standard curve on a grid paper or graph with Excel in class to make sure that it is linear and that you do not need to repeat the experiment. If it plateaus, do not include those data in your graph. Student often are confused by the x-axis in a standard curve. The standard curve sometimes shows absorbance versus amount of substance and other times absorbance versus of concentration of sample. It may be less confusing for some students to work with amounts while doing an assay, especially because dilutions are required. As long as you know the original volume of sample that was used in an assay, determination of amount in the well is easy. Amount of BSA in well = Concentration of BSA solution used for this well (mg/mL)*Volume of the BSA solution used in the assay (mL). Another occasional point of confusion is the distinction between BSA concentrations of the standard solutions (the dilutions you make in eppendorf tubes) and the BSA concentrations in the wells of the microtiter plate. You will make a set of solutions (your standards) in Eppendorf tubes. You will then take 50 uL of each solution and mix with 150 uL of Coomassie reagent in a well in a microtiter plate. After you mix 50 uL of BSA solution with the Coomassie reagent, the BSA concentration in the well is lower (by 4-fold) compared to the BSA standard in the eppendorf tube. When preparing your standard curve, if you express your x-axis as a concentration, be clear about whether it is the BSA concentration in the well in the microtiter plate (after combining protein with Coomassie Reagent) or the BSA concentration of the standard solution you prepared in your Eppendorf tube. Vocabulary BSA, standard, incubate, aliquot, reagent (dye), colorimetric spectroscopy, 96-well plate, microtiter plate, Bradford reagent, Coomassie reagent 10. Safety You must wear safety glasses when conducting the experiment. You must never eat or drink in the laboratory. You will need to wait in line to use the plate reader. Please, be patient when waiting for your turn to use the plate shaker and plate reader. Be gentle with the plate reader and plate shaker, these are delicate instruments. Any observed violations of these rules will result in lower final grade and/or removal from the lab. These safety items are solely the responsibility of the student. 11. Clean up 5 For clean-up, return the remaining original solution to the instructor. Discard dilutions in the sink and eppendorf tubes in the regular trash. Do not throw anything in the biohazard waster. Wash 96-well plates and leave them at the sink to dry. Return pipettors in the correct boxes, the last person puts the boxes in the cabinet in the back of the lab. Dry your ice buckets and place them back in the cabinet. Place all other items where you got them from. Make sure they are clean. Leave your bench ready for the next class to start working. 12. Data Sheet (Homework) Hand in a 1-page data sheet with data and calculations one week after the completion of the Module. Include the names of your group members, date, class, section and instructor name. Provide the data sheet in professional format, include correct units, data labels etc. Do not use excessive significant figures and present your data in tables when possible. Remember to include the following: • Table showing your data. Remember units, label each column and row clearly and precisely. • Standard Curve. You will need to prepare a standard curve by plotting average blank-corrected 564 nm measurements for each BSA standard vs. its concentration in µg/mL (or you can use amounts). • Remember to provide captions for your tables and figures. 6