Jellyfish nervous systems
advertisement

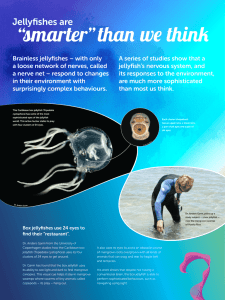
Current Biology Vol 23 No 14 R592 Quick guide Jellyfish nervous systems Takeo Katsuki and Ralph J. Greenspan What are jellyfish? The term jellyfish generally refers to the umbrellashaped gelatinous zooplanktons that belong to Scyphozoa (true jellyfish), Staurozoa (stalked jellyfish), Cubozoa (box jellyfish), and Hydrozoa of Phylum Cnidaria. Their sizes, shapes, and habitats are diverse. Sexually mature jellyfish range from millimeters to meters in diameter, and they can be found almost anywhere in the ocean from the arctic to the tropics and from the deep sea to the shoreline (some even live in freshwater). Despite the substantial variety of their morphologies, some features are shared in common. First, they possess cnidocytes or stinging cells with which they capture prey and protect themselves from predators. Second, they have only one external opening for food intake, waste disposal, and gamete discharge. Third, they are radially symmetric around the single oral/aboral body axis, typically with a symmetry order of four or more. What is their life cycle? The life cycle of jellyfish consists of two main stages: polyp and medusa. The polyp is a sessile form usually stalk-like or funnel-like in shape. Polyps attach themselves to a solid surface and reproduce asexually by budding, dividing, or podocyst formation. When conditions, for example, nutrition, temperature, salinity and lighting, are favorable, polyps produce free-swimming medusae. In most scyphozoans, a single polyp gives rise to multiple immature medusae or ephyrae by horizontal division, a process termed strobilation. In contrast, polyps of Hydrozoa and Cubozoa do not undergo strobilation; rather, a single medusa metamorphoses or buds out from the stalk of a polyp. In general, a medusa has a radially symmetric bell or umbrella. Contraction of the subumbrella muscles squeezes the bell and generates the thrust for swimming. Mature medusae, usually gonochoristic (being either male or female), produce eggs or sperms for sexual reproduction. Fertilized eggs develop into planula larvae, which swim around by means of their cilia until they settle on a solid surface to transform into polyps, and the cycle restarts. The order of the life cycle, however, is not absolute. Some species skip the polyp stage or even revert from medusa to polyp without undergoing sexual reproduction. Do they have brains? No, jellyfish have no single centralized brain. Instead, they have radially distributed nervous systems that are adapted to their unique body plan. Although their nervous system is relatively simple, a common misunderstanding is that all jellyfish have only a diffuse nerve net in which neurons are found homogeneously spread apart. In fact, most jellyfish species show some degree of neuronal condensation that serves as an integrative nervous system. As little is known about the nervous system of stauromedusae, here we focus on scyphomedusae, cubomedusae, and hydromedusae. What do their nervous systems look like? Of the three medusozoan clades, the nervous system of scyphomedusae exhibits the most diffuse organization. There are three major neuronal components that are known to be physiologically and histologically distinct in the Scyphozoa nervous system: rhopalia, the motor nerve net, and the diffuse nerve net (Figure 1A). The rhopalium is the sensory structure that contains ocelli (pigmented photosensitive structures), statocysts (gravity receptors), and pacemaker neurons that set the basic swim rhythm. The neurons in a rhopalium form several morphologically and molecularly distinct populations, some of which are interconnected via commissures, locally exhibiting a bilaterally symmetrical organization (Figure 1B). Most scyphozoans have eight or more rhopalia, the number of which is typically in multiples of four, around the bell margin. The motor nerve net of scyphomedusae is the network of neurons that directly activates muscle contraction in response to the signals from the pacemakers. Neurons of the motor nerve net largely orient randomly and cover the entire subumbrella muscle sheets, and they form chemical synapses at the point of intersection. Because the synapses among these neurons are bidirectional, transmission of excitation is nonpolarized; this allows for throughconduction of the entire nerve net, thus broad, synchronized contraction of the swimming muscles. The diffuse nerve net consists of a neuronal group that generally has immunoreactivity to antibodies against neuropeptides that terminate in arginine-phenylalanine (known as RFamide peptides). Activities of the diffuse nerve net induce marginal tentacle contraction but not muscle contraction on its own. The diffuse nerve net is believed to relay sensory information to the musculature indirectly by affecting the pacemaker activities, and directly by providing modulatory input to the musculature. Are there any more elaborations? The neuronal organization of cubomedusae is similar to that of scyphomedusae in the sense that they have nerve nets and rhopalia (hydromedusae do not have rhopalia); however, its degree of neuronal condensation and integration is in another league. As the name depicts, Cubozoans have a squarish shape with four tentacles and four rhopalia. Each rhopalium contains six eyes of four different types, two of which (the upper lens eye and the lower lens eye) are highly developed image-forming eyes with cornea, pupil, lens, and retina, much like our own (Figure 1C). Several histologically and anatomically discrete neuronal populations are found, most of which are situated in a bilaterally symmetrical manner with commissures connecting two sides of the rhopalium. Neighboring rhopalia are connected via a ring nerve (RN in Figure 1C), a condensed array of neuronal tracts that is believed to integrate the swim system, the visual system, and the tentacle system. The ring nerve consists of three anatomically different types of neurites that are interconnected by chemical synapses. In some parts of the ring nerve, neurons are divided into compartments by extensions from epithelial cells, which may provide electrical insulation between neurites. Do all jellyfish depend on nerve nets? No, some hydromedusae lack nerve Magazine R593 nets. Their neurons are confined to the inner and outer nerve rings that run around the bell margin. These nerve rings consist of multiple parallel neuronal pathways that process different sensory inputs such as light, gravity, and touch. Pacemaker neurons are also located in the nerve rings. One Hydrozoa species, Aglantha digitale, has been shown to have at least 14 physiologically distinct pathways in the nerve rings (Figure 1D). In hydromedusae, broad, synchronized contraction of subumbrella muscles is achieved by electrical coupling of muscles through gap junctions (gap junctions are found only in Hydrozoa). In Aglantha, however, besides the electrical coupling among muscles, motor commands around the ring nerve are transmitted to the muscle sheets via neuronal tracts that run up radially and then laterally along the subumbrella muscles. Thus, jellyfish from different clades, or even within a clade, provide a broad spectrum of strategies for neuronal integration. What behavioral repertoires do they have? Known behaviors of scyphomedusae include sun compass navigation, diel vertical migration, avoidance of low salinity, escape from contacts with predators, and formation of aggregates. Although the neuronal bases for these behaviors are poorly understood, scyphomedusae appear to respond to various stimuli such as gravity, light, salinity, touch, and chemicals. Cubomedusae demonstrate a richer repertoire of vision-based behaviors, as expected from their state-of-theart light-sensing organs. Tripedalia cystophora look up through the water surface with their upper lens eyes, and direct themselves to the mangrove canopy, their preferred habitat. Laboratory studies have shown that they use visual cues most likely through their lower lens eyes to locate light shafts and to avoid obstacles as they swim. It is presumed that they do the same thing as they navigate through the tangled underwater landscape of mangrove swamps to find the light shafts where their prey gather. These behaviors require not only accurate vision but also precise control of speed and direction of swimming. This is achieved by modulating pulse frequency, creating an asymmetry in the opening of the A B te LC Rhopalia O-D in A-L O-C A-L O-C bs O-P MNN DNN D C MG Rhopalia P ULE RG RN LLE Tentacle TG Current Biology Figure 1. Nervous systems of three medusozoa clades. (A) Neuromuscular system of Aurelia sp.1 (Scyphozoa) ephyra stained with phalloidin (green) and antibodies against tyrosinated tubulin (magenta). (B) Schematic diagram of rhopalium nervous system consisting of terminal (te), intermediate (in), and basal domains (bs). Tyrosinated tubulin and taurin immunoreactive neurons (green) are connected to motor nerve net. FMRFamide immunoreactive neurons (blue) are connected to diffuse nerve net. GLWamide immunoreactive neurons (red) connect the intermediate segment and basal segment. LC, lithocyst (statocyst); O‑C, oral-central sensory cells; O-D, oral-distal sensory cells; A-L, aboral-lateral group. (Adapted with permission from Nakanishi et al. (2010).) (C) Tripedalia cystophora showing two rhopalia and a ring nerve (RN). (Adapted with permission from Skogh et al. (2006).) Inset shows a higher magnification of a rhopalium with a lower lens eye (LLE), an upper lens eye (ULE), two slit eyes, and two pit eyes. (Adapted with permission from Nilsson et al. (2005).) (D) Wiring diagram of Aglantha digitale nervous system. For simplicity, only the swimming system is shown, and tentacle giant axon (TG, thick pink), motor giant axon (MG, thick red), pacemaker system (P, green), ring giant axon (RG, blue) are labeled. (Adapted with permission from Mackie (2004).) bell, and delaying contraction on one side of the bell in response to light changes. One species of Cubozoa, Carybdea sivickis, has been reported to engage in courtship rituals, in which a male grabs a female with its tentacles and transfers spermatophores onto her tentacles. Male Carybdea selectively approach females with conspicuous velar spots, a putative sign of sexual maturation, but it is not clear what sensory modalities are involved in the recognition. What is known about neuronal control of behaviors? One of the most extensive studies of the neuronal mechanisms of jellyfish behavior has been done on the control of swimming in the hydromedusa Aglantha digitale. Atypically for hydromedusae, Aglantha has two different swimming modes: slow swimming and escape swimming. Slow swimming is their normal mode, and is mediated by relatively regular and weak propulsion of the bell governed by pacemaker neurons in the nerve rings. Escape swimming produces a much stronger contraction of the bell, and is triggered when one of the giant neurons in the nerve rings (ring giant) is excited by mechanical stimulation of the tentacles. In both swimming modes, neuronal activity in the nerve ring is transmitted to the muscles via the motor giants that run up the bell. Current Biology Vol 23 No 14 R594 The question then is how the same neurons convey two different sorts of information. The answer is that motor giants are capable of generating two types of action potentials, small/slow calcium spikes and large/fast sodium spikes, depending on the degree of depolarization it receives. As opposed to the input from the ring giant, the input from the pacemaker is too low to trigger the sodium spikes, thus only inducing slow swimming. In addition to speed control, Aglantha can steer the direction of swimming by changing the shape of the bell opening, the velum. Many Hydrozoa species, including Aglantha, can move individual tentacles to deliver captured prey to the mouth, which requires coordinated directional movement of a tentacle, velum, and manubrium. What is interesting about jellyfish nervous systems? Being an outgroup to Bilateria with complete nervous systems, jellyfish have been attractive models for studying the evolution of nervous systems. For example, by comparing the expression of genes involved in the development of sense organs with that of Bilaterians, one could ask the evolutionary origin of sense organs. From a physiological point of view, the relatively primitive architecture and behavior of jellyfish provide an opportunity to address how sensory inputs and internal information are integrated to produce coordinated motor outputs. Intriguingly, despite the simplicity of their neuronal organization, jellyfish appear to have the full battery of molecular machinery for neurotransmission and neuromodulation (for example, ion channels, traditional neurotransmitters, peptides, amino acids, small molecules, and their receptors). It remains largely unknown how this molecular variety contributes to the function of jellyfish nervous systems. Jellyfish have adapted to a wide range of ecological situations by developing different sizes, shapes, and rigidity of the bell and tentacles, all of which significantly affect the mechanics of the animals and their interaction with the fluid environment. Consequently, the nervous system must also be matched to the animal’s physical properties. Comparative approaches in jellyfish might provide insights into how neuronal networks can create a diverse range of functions from a limited degree of complexity. It is also of interest that many medusozoans are capable of regenerating large portions of their nervous system, and appear to use different combinations of stem cells and committed cell types. What resources and tools are available for studying jellyfish nervous systems? Taking advantage of the effectively two-dimensional, transparent, gelatinous body, various physiological, histological, and pharmacological approaches have been employed to examine cellular and network-level mechanisms in jellyfish. One of the most important resources lacking at present is a set of genetic and molecular tools. For the Hydromedusan species Clytia hemisphaerica and Podocoryne, expressed-sequence tagged (EST) databases are publicly available through NCBI dbEST, and genome sequences are forthcoming. Genome projects for Aurelia sp.1, and Cladonema pacificum are also under way. Efforts are being made to develop molecular tools, including RNA interference (RNAi), morpholinoantisense oligonucleotides, and transgenesis. Arrival of such resources should establish the jellyfish as a valuable model to study function, development, and the evolutionary origins of nervous systems. Where can I find out more? Arai, M.N.A. (1996). Functional Biology of Scyphozoa. (Springer). Satterlie, R.A. (2011). Do jellyfish have central nervous systems? J. Exp. Biol. 214, 1215–1223. Costello, J.H., Colin, S.P., and Dabiri, J.O. (2008). Medusan morphospace: phylogenetic constraints, biomechanical solutions, and ecological consequences. Invertebrate Biol. 127, 265–290. Garm, A., Oskarsson, M., and Nilsson, D.-E. (2011). Box jellyfish use terrestrial visual cues for navigation. Curr. Biol. 21, 798–803. Houliston, E., Momose, T., and Manuel, M. (2010). Clytia hemisphaerica: a jellyfish cousin joins the laboratory. Trends Genet. 26, 159–167. Mackie, G.O. (2004). Central neural circuitry in the jellyfish Aglantha: a model ‘simple nervous system’. Neurosignals 13, 5–19. Nakanishi, N., Yuan, D., Hartenstein, V., and Jacobs, D.K. (2012). Evolutionary origin of rhopalia: insights from cellular-level analyses of Otx and POU expression patterns in the developing rhopalial nervous system. Evol. Dev. 12, 404–415. Nilsson, D.-E., Gislén, L., Coates, M.M., Skogh, C., and Garm, A. (2005). Advanced optics in a jellyfish eye. Nature 435, 201–205. Skogh, C., Garm, A., Nilsson, D.-E., and Ekström, P. (2006). Bilaterally symmetrical rhopalial nervous system of the box jellyfish Tripedalia cystophora. J. Morphol. 267, 1391–1405. Kavli Institute for Brain and Mind, UCSD, La Jolla, CA 92093-0126, USA. E-mail: rgreenspan@ucsd.edu Primer C4 photosynthesis Elizabeth A. Kellogg The number of humans on earth is increasing, generating concerns about food security and spurring efforts throughout the world to increase the productivity of crops. If a way could be found to increase the yield of crops by, say, 20%, it would have immense impact on global food supplies. Fortunately, evolution has already crafted such a mechanism, known as C4 photosynthesis. The C4 pathway is in effect a turbocharger for the more conventional C3 pathway. Just as a turbocharger improves performance of an engine by forcing more air into the manifold, C4 improves photosynthetic performance by forcing CO2 into the standard C3 photosynthetic apparatus. The added efficiency of this mechanism is obvious at a global level. Only about 3% of flowering plant species use the C4 pathway, but this relative handful of species account for 23% of the carbon fixed (primary productivity) in the world. The pathway occurs in several of the world’s major crops, notably maize (corn) and sorghum, and in many of the species in use as biofuels, most importantly sugar cane; all of these are grasses in the family Poaceae. If a major C3 crop such as rice could be bred to use the C4 pathway, the economic impact would be immense. This Primer will summarize the current state of knowledge about C4 photosynthesis and highlight one of its enduring mysteries — despite years of study and deep understanding of its physiology, biochemistry and ecology, the trigger for development of C4 remains unknown. As noted in the next section, the pathway has arisen many times in recent evolutionary history. The physiological, biochemical, anatomical, and genetic data described in the subsequent sections all point to a process whose complexity appears at odds with the apparent ease with which C4 originates. Some hints about the controls come from the study of photorespiration, so-called C2 photosynthesis, but, as outlined