Energy WebQuest
advertisement

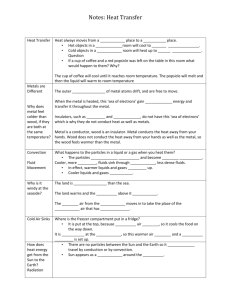
Energy, Heat, Work, Temperature Web Quest PURPOSE In this web quest you will do a virtual lab, a discovery lab, watch videos and read information online in order to answer three central question of this lesson: Should you use wooden spoon or metal spoon when making candy? If a metal spoon is to be used what kind of metal would be best? If a wooden spoon is to be used what kind of wood would be best? This activity is appropriate for an introductory chemistry course. During this lesson, you will be introduced to the concepts of energy, heat, work, temperature and specific heat capacity. For the point value of this web quest go to the last page and look at the grading rubric. CONTEXT For this lesson it is important for you to begin to appreciate the importance of science and how it relates to culinary practices. Sometime chefs follow certain culinary practices based on habits or what they were told and not the scientific behind that culinary practice. Culinary practice another words is cooking skills. The ultimate goal of this lesson is for students to answer the central question: What substance should a spoon be made of when it is used in candy making and the science behind the correct culinary practice? In order to do this, students will watch two videos and answer questions on a worksheet, do a virtual lab to determine the specific heat of metals, a lab activity to determine the specific heat of types of wood as well as read an online article about the types of spoons used in cooking. Begin the activity by reading these two articles at http://www.thekitchn.com/candymaking-basics-do-you-use-69509 and at http://www.reluctantgourmet.com/wooden-spoons-a-must-in-every-kitchen/ Answer the following questions about these articles on you own notebook paper with the following title at the top of your notebook paper: “What type of spoon and why?” 1. What kind of spoon does food scientist Harold McGee suggest in cooking and why? 2. So who do we trust: the Mighty McGee or our baking-veteran chef? What do you say? (Note: This is your own opnion) 3. After reading the other article what other factors are important about the type of spoon used? MOTIVATION You will now answer questions for the following two worksheets over three videos you are to watch. The links to these videos are at the top of each page and one is embedded in a question. You should print out the worksheets on the next four pages before watching the video. You may and should put your answer to these questions on the worksheets you have printed off and not on your own notebook paper! VIDEO WORKSHEET #1 Watch the Crash Course Chemistry #17 “ Energy and Chemistry” by Professor Hank o According to Professor Hank “everything” is …………………….. o The energy that a trebuchet contains can be put into three boxes labeled o Circle the box that has the energy most difficult to access. o Put the word motion under the box that the energy comes from the movement of the atoms and molecules. o Write the word fire under the box that would give us this form of energy if the bonds are broken by burning the trebuchet o What is gravitational potential energy? o What does the first law of thermodynamics and/or the law of conservation of energy state? o What is thermodynamics? o Although tough to define what is the word meaning the capacity to do work or produce heat? o IF an object has a force acted upon it, but does not move then then no ……………….. is said to be done. o Can object contain heat? Explain. o How is work symbolized? o How is heat symbolized? (I am like Professor Hank just deal with it!) o When talking about energy what two parts is energy divided into? o Who decides where the division is made? o How do we represent the change of energy equal heat plus work? o What is the sign value (+ or -) if work is done to set the trebuchet? o In the above case who is the system and who is the surroundings? o What is the sign value (+ or -) when the trebuchet is fired? o The energy stored in chemical bonds is what type of energy? o IF Professor Hank was to burn the trebuchet the reaction would be said to be an …….. o When energy goes into a system then the reaction is said to be an ……… o What is the equation for the reaction in your car showing the above type of reaction? VIDEO WORKSHEET #2 Watch part of the Crash Course Chemistry #19 “ Calorimetry” by Professor Hank o For this video, only questions that are important for our activity will you be answering questions, but watching the entire video is highly suggested to get the concept of calorimetry!! o Write the definition for calorimetry, but just drop the words a chemical reaction with and replace with of an energy exchange between two substances o What does a calorimeter look like? o Why is insulation important for a calorimeter/ o What is the thermometer measure? (Hint: Is one temperature reading made or two?) o You may stir with our electronic thermometers! o What is the formula used to find the heat change? What does each letter represent, please give a definition of s? (NOTE: Many chemist including me use c instead of s, one of the few times Professor Hank and I disagree and we will use q instead of H like he explained) o Does adding equal amount of heat to any substance cause the same temperature change to each substance? o How does the specific heat capacity of water and the specific heat capacity of metals compare to each other? (HINT: Look at your answer to the previous question!) o Not part of this video but, what sensations do feel when you pick up a metal spoon from your silverware drawer? Explain the sensation. o How do we (chemist) find specific heat capacities? o Why is mass important? o (True or False) Heat and temperature are the same thing. Explain how a calorimeter relates these two terms. o Are we doing physics or chemistry? Does it really matter? o What is the specific heat capacity of water? o Why is the heat a + number after doing the calculations? o At this point he is talking about the calorimeter determining heat for a chemical reaction and we are going to be dealing with hot objects going into cold water and using this to determine specific heat so you may stop watching this video or watch it is up to you. o Not using this video, but using the video Specific Heat of a Metal Lab or Calorimetry Lab Handout describe in your own words, how a coffee-cup calorimeter can be used to determine the specific heat capacities of metals and/or different types of wood? As part of this answer you should include what measurements need to be made. DEVELOPMENT Go to the webpage http://oceanservice.noaa.gov/education/pd/oceans_weather_climate/media/specific_heat.swf Your screen should like this follow the instructions tab in the upper right hand corner. After allowing this web simulator to play, which “stuff” has a higher specific heat, the water on the left or the soil on the right. Your answer written below must include data from the experiment to support your claim. You may either print this page out and put your answer below OR put you answer on your own notebook paper. If you are doing this on your own notebook paper put the following title at the top of your notebook paper: “Development, Water or Soil?” VIRTUAL LAB Before doing this lab you may want to watch this YouTube video first AND then use the web simulator to complete the following lab: DIRECTIONS 1. Click on the metal "SILVER" in the top left of the screen. 2. Set the parameters to the following: mass of metal = 120 g temp of metal = 220oC mass of water = 50 g temp of water = 20oC 3. Record the specific heat of the metal and the water. 4. Click START. 5. Record the final temperature of the water and the metal. 6. Repeat the experiment for the remaining five metals, two of which are unknowns. 7. Complete the following table. CALCULATIONS/ANALYSIS (SHOW ALL WROK ON YOUR OWN NOTEBOOK PAPER!) 1. For each metal calculate the heat lost by the metal using q = m c (Tf-Ti). This answer will be in Joules and will be negative because the heat is lost by the metal. 2. For each metal, calculate the heat gained by the water using q = m c (Tf-Ti). This answer will be in Joules and will be positive because the heat is gained by the water. 3. Compare the answers for the heat lost and heat gained for each metal/water combination. Do they agree? Should they? Justify your answer. If they do not agree, what are some sources of error for this experiment in the real laboratory? 4. Calculate the specific heat for the unknowns. Using a table of specific heat values, find a likely answer to the question of the identity of the unknowns. 5. Which metal would be best for a spoon to be made out of based solely on the specific heat capacities of all the metals shown in this lab (this list to choose from includes the known metals and the unknown metals shown in the virtual lab)? DISCOVERY LAB Your teams goal for this lab is to determine the specific heat of a wood sample you have been given. At the end of this lab you are expected to post your specific heat value you have obtained. How you accomplish this is up to you, but you must have my OK for your procedures before beginning. You will also record the specific heat of other types wood done by your classmates. Lab Discussion Some things you and your lab partners should keep in mind: You will work in a group of no more than four students You will have two days to do the lab and this includes all pre-lab preparation You must have in writing before beginning any lab procedure: o A statement of purpose o Any mathematical equation that you will be using o A set of bulleted procedures o A list of equipment and materials need o A data table to record all qualitative and quantitative observation Wear eye protection at all times! Have a calculation section showing the work to obtain any number not directly measured in lab A conclusion reporting your results Check the lab grading protocol for any and all point values Good luck and remember TO THINK! You will be responsible for getting other groups results for the other type of wood that you were not assigned so you can answer the next bullet Determining which wood type you think is best AND FINALLY which type of spoon you think is best! Supported with “hard data” (HINT: Go back to the original purpose and answer the essential questions supported with what you have learned through this activity) Lab 4 Specific Heat of Wood Lab Partner 1: _______________________________ Lab Partner 2: _______________________________ Lab Partner 3: _______________________________ Lab Partner 4: _______________________________ Item Points/Out of Name & Partner /1 Date Experiment Started /1 Title of Experiment /1 Appropriate Purpose /4 Mathematical Equation Used /2 Procedures /10 Equipment and Materials List /6 Data Table (with appropriate information) /10 Data Table (Specific Heat of other Groups) /5 Calculations /15 Conclusion (Statement of Results) /15 Late (10% for first day: second day late no credit) TOTAL /70 Energy, Heat, Work, Temperature Web Quest Grading Rubric Student Name: _______________________________ Item Points/Out of Context Three Questions /7 VIDEO WORKSHEET #1 /30 VIDEO WORKSHEET #2 /25 Development /7 Virtual Lab /51 Discovery Lab /70 TOTAL /190