Chapter 13 Chapter 13 Nuclei
advertisement

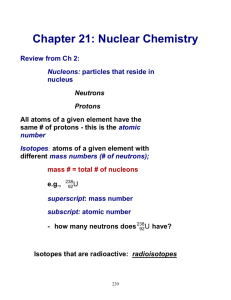
Chapter 13 Nuclei Mass is converted into energyy accordin according to the formula E = m c2 A uranium nucleus (atomic number 92, mass number 238) emits an α-particle particle and the resultant nucleus emits a β-particle. particle. What are the atomic and mass numbers of the final nucleus (a) atomic number umber = 91 (b) mass number = 234 In the uranium radioactive series the initial nucleus is 92U238 and final nucleus is 82Pb206 when the uranium nucleus decays to lead, the number of α-particles emitted is 8 and the number of βparticles emitted is 6. When Boron nucleus (5B10) is bombarded by neutrons, α-particles particles are emitted. The resulting result nucleus is of the element 3Li.6, and has the mass number 6. Atoms having the same but different Atomic number, mass number are called isotopes. Spontaneous ous fission was discovered by Flerov and Petrzhak. A radioactive source has a life of 3 hours. A freshly prepared sample of the same emits radiation 16 times the permissible safe value. The minimum time after which it would be possible to work safely with the source is 12 hours. Properties of α,β and γ-rays The comparison of the properties of α, β and γ-rays are shown below in the table: Properties α-rays β-rays Nature Helium Fast moving Electromagnetic nucleus electrons waves or photons Nature of charge Positive Negative No charge Magnitude of charge 3⋅2 × 10-19 1⋅6 × 10-19 Zero coulomb Mass γ-rays coulomb 6⋅6 × 10-27 9⋅1 × 10-31 kg Rest mass zero kg Velocity Between 1⋅4 1% to 99% 3 × 108m/sec × 107 m/sec of velocity of to 2⋅2 × light 107 m/sec Effect of electric and Deflected Deflected Not deflected magnetic fields Range 2⋅7 to 8⋅62 5 mm of Al 30 cm of iron cm in air or or 1 mm of 1/100 mm lead of Al Penetrating power Ionizing power minimum maximum 100 times of 1000 times of αα-rays rays Lesser Minimum Distinction Between fission and fusion Fission 1. Fusion Huge amount of Huge amount of energy is energy is liberated. liberated 2. A heavy nucleus is Two lighter nuclei are fused split up into two (combined) together nuclei 3. This process This process is only possible at possible even at very high temperature room temperature 4. The links of this The links of this process are process are protons neutrons. 5. The energy nucleon is per The energy per nucleon is 6⋅75 200 MeV. MeV. The half life of a certain radio isotope is 10 minutes. The number of radioactive nuclei at a given instant of time is 108. Then the number of radioactive nuclei left 5 minutes later would be (1/√2) × 108. A sample contains 10 gm of radioactive material, the half life of which is two days. After 32 days, the amount of sample is less than 1 mille gram. A radio isotope has half life f 5 years. The fraction of the atoms of this material that would decay in 15 years will be 7/8. The radius of a nucleus determined by electron scattering is found to be slightly different from that determined by alpha-particle scattering. The nature of the binding energy (per nucleon) curve shows that exothermic nuclear reactions are possible, when two light nuclei fuse or when a heavy nucleus undergoes fission into nuclei with intermediate mass. For fusion, the light nuclei must have sufficient initial energy to overcome the coulomb potential barrier. That is way fusion requires very high temperatures. Electrons and positron are a particle-antiparticle pair. They are identical in mass; their charges are equal in magnitude and opposite. (It is found that when an electron and a positron come together, hey annihilate each other giving energy in the form of gamma-ray photons.) A free neutron is unstable (n → p + e-+ v ). But similar free proton decay is not possible, since a proton is (slightly) lighter than a neutron. The more readily fissionable isotope of uranium has an atomic mass of 235. When the mass equal to one a.m.u. is converted completely into energy, the energy produced is 1⋅5 × 10-10 joule. One atomic mass unit is equal to 1⋅67 × 10-27 kg. If the end A of a wire radiates α-particles and the other end B radiates β-particles then current will flow from A to B. The radioactive decay rate of a radioactive element is found to be 103 disintegrations per second at a certain time. If the half life of element is one second, the decay rate after one second is 500 and after 3 second is 125. Mass of uranium in an atom bomb is termed as Critical mass. How many electrons, protons and neutrons are there in a nucleus of atomic number 11 and mass number 24. (a) Number of electrons 0. (b) Number of protons 11 (c) Number of neutrons 13 On the atomic scale, mass is measured in atomic mass units (u). By definition, 1 atomic mass unit (l u) is 1/12th mass of one atom of 12C; 1 u = 1. 660563 × 10-27 kg. A nucleus contains a neutral particle called neutron. Its mass is almost the same as that of proton. The atomic number z is the number of protons in the atomic nucleus of an element. The mass number A is the total number of protons and neutrons in the atomic nucleus; A = Z+N; Here N denotes the number of neutrons in the nucleus. A nuclear species or a nuclide is represented as , where X is the chemical symbol of the species. Nuclides with the same atomic number Z, but different neutron number N are called isotopes. Nuclides with the same A are isobars and those with the same N are isotones. Most elements are mixtures of two or more isotopes. The atomic mass of an element is a weighted average of the masses of its isotopes. The masses are the relative abundances of the isotopes. A nucleus can be considered to be spherical in shape and assigned a radius. Electron scattering experiments allow determination of the nuclear radius; it is found that radii of nuclei fit the formula R = R0 A1/3 Where R0 = a constant = 1.2 fm. This implies that the nuclear density is independent of A. it is of the order of 1017kg/m3. Neutrons and protons are bound in a nucleus by the short- range strong nuclear force. The nuclear force does not distinguish between neutron and proton. The nuclear mass M is always less than the total mass, ∑m, of its constituents. The difference in mass of a nucleus and its constituents is called the mass defect, ∆M = (Z mp + (A – Z)mn) – M Using Einstein’s mass energy relation, we express this mass difference in terms of energy as ∆Eb = ∆M c2 The energy ∆Eb represents the binding energy of the nucleus. In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant, about 8 MeV/nucleon. Energies associated with nuclear processes are about a million times larger than chemical process. The Q-value of a nuclear process is Q = final kinetic energy-initial kinetic energy. Due to conservation of mass-energy, this is also, Q = (sum of initial masses – sum of final masses)c2 Radioactivity is the phenomenon in which nuclei of a given species transform by giving out α or β or γ rays; α-rays are helium nuclei; β-rays are electrons, γ-rays are electromagnetic radiation of wavelengths shorter than X-rays; Law of radioactive decay : N (t) = N (0) e-λt Where λ is the decay constant or disintegration constant. The half-life T1/2 of a radionuclide is the time in which N has been reduced to one-half of its initial value. The mean life τ is the time at which N has been reduced to e-1 of its initial value T= λ = τ ln 2 Energy is released when less tightly bound nuclei are transmuted into more tightly bound nuclei. In fission, a heavy nucleus like U breaks into smaller fragments, e.g., U + n → Sb + Nb + 4 n Physical Symbol Dimension Units Remarks Quantity Atomic mass unit [M] u Unit of mass for expressing atomic or nuclear masses. One atomic mass unit equals 1/12th of the mass of 12C atom. Disintegration or λ [T-1] s-1 [T] s decay constant Half-life T1/2 Time taken for the decay on onehalf of the initial number of nuclei present in a radioactive sample. Mean life τ [T] s Time at which number of nuclei has been reduced to e-1 of its initial value Activity radio- of a R active [T-1] Bq Measure of the activity of a radioactive source. sample Radioactivity is an indication of the instability of nuclei. Stability requires the ratio of neutron to proton to be around 1:1 for light nuclei. This ratio increases to about 3:2 for heavy nuclei. THANK YOU