Mycology for Beginners
advertisement

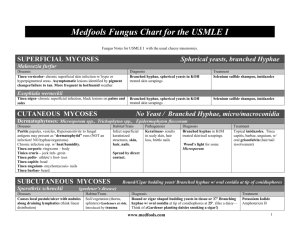
Mycology for Beginners Louise Hafner Ann McPhee Mycology Study of fungi Ubiquitous – commonly found Soil around roots of plants Foliage & thorns Skin, mouth, intestine – animals, birds, insects Water - natural water courses Air – spores 1.5 million species with 70000 described Benefits of fungi Food source – mushrooms, truffles Food production bread, cheese, alcoholic drinks, soy sauce Production Vitamin C, Citric & Oxalic acids Production antibiotics penicillin & cephalosporins Decomposition waste materials Biological control – pests & disease Harmful effects Destruction of materials - wood Spoilage - stored food Spontaneous combustion – hay & peat Mycotoxins – aflatoxin - liver damage Destruction of crops – potato blight Phytophtora infestans Infect animals – Pythium, Mortierella Infect man – 200 human pathogens Human infections 8-10 dermatophytes (30) 15 yeasts (650) 30 moulds (30000) History Mycetoma & thrush described 1835 Bassi - muscardine in silkworms 1839 Schoenlein & Gruby – favus (scalp infection) 1839 Lagenbeck – Candida albicans Preceded Koch & Pasteur 1850-60 1910 Sabouraud - “Les Teignes” 30yr study dermatophytes Histoplasma, Coccidioides, Chromoblastomycosis Norman Conant & Chester Emmons - taxonomy Fungi Three Domain Classification Domain Bacteria ) contains the eubacteria (true bacteria) Domain Eucarya protozoa, algae fungi, plants & animals Domain Archaea contains the archaebacteria (primitive bacteria) ‘Brock Biology of Microorganisms’ 12th edition.T. Madigan et al, Definition Eukaryotic Membrane bound organelles Nuclei, mitachrondria, golgi apparatus, lysosomes Spore bearing & reproduce by sexual & asexual means Differ from bacteria – prokaryotic Heterotrophic Lack chlorophyll - not autotrophic Saprophytes (saprobes) on dead material or parasites on living Rigid cell walls – non motile Resistant antibacterial antibiotics Taxonomy – „Classical‟ Based on spore production Reproduce sexually – meiosis – teleomorph Zygomycotina Ascomycotina Basidiomycotina Reproduce asexually – mitosis - anamorph Deuteromycotina Artificial division – teleomorph unknown Taxonomy – „Molecular‟ Characteristic morphology - well-known pathogens easily identified Development of molecular methods Need to balance both approaches Need a timely & clinically useful report - clinician can diagnose and treat Clinical mycologists use anamorph names Molecular mycologists use teleomorph Structure - Yeast Unicellular Reproduce by budding - blastoconidia Colony mucoid +/- pseudohyphae +/- true hyphae Basidiomycete – Cryptococcus Ascomycete - Candida Structure - Mould Filaments – hyphae Mass hyphae makes a mycelium or thallus Reproduce by spores or conidia Sporulation enables identification Dimorphic fungi - Hyphae 25-30 C Yeast 35-37 C Hyphae – non-septate Coenocytic Thin walled Broad hyphae Sparse septa Zygomycetes Primitive fungi – hyphal damage = death Grows & spores rapidly Hyphae - septate Contents move between compartments If damaged, pores plugged by Woronin bodies Prevents death whole strand Hyphomycetes Basidiomycetes Reproduction Asexual – mitosis – anamorph - synanamorph Conidia – production, morphology & arrangement Culture – texture, topography, pigment, growth 37 C Identifies fungus Majority medically important fungi Hyphomycetes ( maybe Ascomycetes) Hyalohyphomycetes, Phaeohyphomycetes (Dematiaceous) Dermatophytes (Fungi imperfecti), Dimorphic fungi Reproduction Sexual – teleomorph Heterothallic – 2 different thalli Homothallic – single thallus Methods - meiosis cytoplasmic fusion-plasmogamy fusion two nuclei-karyogamy genetic recombination Zygospores Ascospores Basidiospores Mycosis - fungal disease Superficial: mild infection skin or hair shaft Cutaneous: invasion keratinised tissue Subcutaneous: penetration beneath skin Systemic – deeper infection – tissue/blood Other diseases Allergic reactions Inhalation mould spores - Farmers lung Aspergillus sp. in grain stores, haystack, silos Hypersensitivity – Dermatophytid or „id‟ reaction sterile blisters on hands can diagnose tinea pedis Other disease Toxicity – mycotoxins Mushrooms - Amanita phalloides - death Amanita muscaria (fairy toadstools) Aflatoxin (Aspergillus flavus) in peanuts - liver cancer Ergotamine - hallucinogenic - rye grain Clinical Mycology E.J. Anaissie, M.R. McGinnis & M.A.Pfaller Pathogenicity Accidental exposure Infection not needed for propagation except a few dermatophytes Factors other than pathogenicity host‟s immune status Very few fungi can infect healthy human but needs unique enzyme capacity thermal dimorphism can block cell-mediated immune defences Pathogenicity Barriers Temperature - fungi prefer <37ºC Redox potential - living tissue lower Cellular defences – lytic activity Non-specific Surface secretions & normal flora Healthy skin & mucosa Pathogenesis of indoor fungi diseases Michael. R. McGinnis 2004 ISHAM Medical Mycology, 42,107-117 MVOC – microbial volatile organic compound VOC – volatile organic compounds FVOC – fungal volatile organic compounds Mycotoxins Any/all can contribute to „sick building syndrome‟ Epidemiology Infection may be acquired Community Person to person contact Environmental exposure Hospital - ICU, organ transplant, oncology patients Candidiasis - IV catheter lines Aspergillosis – dust (construction), pot plants, vents Laboratory Accidental cutaneous inoculation Inhalation of spores Laboratory Mould form dimorphic fungi - highly infectious Occasional isolation Australia Care with Sporothrix & Histoplasma cultures Clinical & travel notes - Coccidioides, Blastomyces Use biohazard cabinets for handling cultures Kwiambal National Park Images from Kaminski‟s Digital Image Library of Medical Mycology and SNP Image Library l.hafner@qut.edu.au ann_mcphee@snp.com.au Don’t panic DON’T PANIC it’s only fungus Louise Hafner Ann McPhee Mycology • • • • • • Fungal microscopy & culture requested Rare bacterial isolates Chloramphenicol and gentamicin in media Nocardia & Mycobacteria spp.- occasional Specimens – Tissues, Fluids Skin & Nail scrapings, Hair pluck Isolate – Yeasts,Dermatophytes,Moulds Microbiology • Bacterial microscopy & culture requested • Fungi grow 35-37ºC - aerobic conditions • Any media except MacConkey (bile salts) • May not sporulate on Blood agar 35ºC • No typical features - difficult to identify • Occasional dermatophyte isolated • Occasional mould isolated Why? • • • • • • • Appearance of lesion deceptive Lesion may be very inflamed Not typical tinea rash/ringworm Kerion- inflammatory infection (scalp) Zoophilic & Geophilic dermatophytes Lesion may be a subcutaneous cyst Exudate swabbed or tissue biopsied - fungal culture not requested Immune Response - Cutaneous • Allergic response varies – fungi & patient • • • • Blisters/vesicles/pomphlox/bullae often clinical notes - impetigo ‘id’ reaction large blisters - palms Sterile blisters - check 4-5th toe web T.pedis Topical use of corticosteroids - alters clinical presentation Immune Response • • • • • • • Immunocompromised - HIV Widespread use of cortisones in medicine Treatment regimes - cancer – asthma auto-immune syndromes - organ transplants Diminished immune system Diabetes - poor circulation - age Better treatment - Better quality of Life Outdoors - exposure to environmental fungi The routine procedure • Most fungi grow 2-5 days 35ºC • Leg ulcer or foreign body or cyst or nodule • Fusarium sp. or Curvularia sp. or ……. • PDA (Potato Dextrose Agar) inoculated • Skin rash, blisters, kerion - dermatophyte • DTM (Dermatophyte Test Media) inoculated Significance • • • • • • • How do you know? Clinically significant fungi grow at 35C May grow faster than at 30C Grow on most media Not MacConkey (bile) or anaerobic No typical features Check the Gram stain for fungal elements The routine procedure • • • • Leucocytes present & no bacteria isolated at 2 days Culture plates kept 5 days Check Gram Stain – rare bacteria, hyphae, yeast cells Hyphae - gram variable Dermatophyte Hyphomycete The Gram Stain • • • • • • • • Gram colour is not important but morphology is Important to recognise the fungal elements Validates growth on plates May only see occasional strand of hyphae Check Giemsa slide if made Check wet preparation - pigmented hyphae/yeast cells Information may indicate the type of fungi Aid selection of antifungal treatment Microscopy • • • • CFW-KOH (Calcofluor White- Potassium Hydroxide) Hyphae ?septate Gram stain Hyphae, Yeast cells +/- pseudohyphae Wet Preparation: Pigment Broad hyphae/few septa: Zygomycete Yeasts • Candida Oval cells narrow based buds Pseudohyphae +/- • Malassezia Round cells - broad based buds No pseudohyphae (commensal) No growth except lipid media Cryptococcus CSF,Blood,Urine,Tissue,Lung,Skin • Cryptococcus Round budding (viable) cells Capsules Gram stain variable - yeast/capsule No pseudohyphae Body Fluids • • • • • • Large volume fluids Dialysis, Pleural, Eyewash Cassettes Centrifuge then cytospin Reconstitute deposit in saline Protein hinders drying, adherence & staining Gram/Giemsa check edge Hyphae may spin to edge ENT- Ear Nose & Throat • • • • • • • Ear & Nasal Sinus/Antrum infections Large spectrum of fungi involved Hyphae seen in wet prep & Gram Yeasts in ear ?Malassezia - commensal Pigmented conidia or erythrocytes? Aspergillus niger? Throats usually Candida infections HIV patients ? Candida dubliniensis Acknowledgements Microbiology Scientists Check gram stains Read clinical notes Read between the lines in the notes Follow-up with clinician? Subculture to PDA and/or DTM Keep plates an extra few days Images from Kaminski’s Digital Image Library of Medical Mycology and SNP Image Library l.hafner@qut.edu.au ann_mcphee@snp.com.au Dermatophyte Identification Louise Hafner Ann McPhee Dermatophyte infections - genera Microsporum Skin Hair Rough walled macroconidia - distinctive Microconidia may be present Epidermophyton Skin Nail Smooth walled macroconidia - distinctive No microconidia Trichophyton Skin Hair Nails Microconidia present - distinctive Smooth walled macroconidia present Martha E Kern Medical Mycology Ecological groups Anthropophilic Trichophyton rubrum parasitic on man only Zoophilic Microsporum canis parasitic on animals human infection - contact with animals Geophilic Microsporum gypseum inhabit soil – keratin debris infection - contact with soil Geographical distribution T. rubrum Worldwide – troop movements, migration, social habits, rapid world travel T. tonsurans Southern Mediterranean then sailors to Mexico, Northern Sth. America & Carribean 1950‟s Hispanic migration North America Mild – Hispanics Chronic - Europeans Severe - Negro population Host specificity T. concentricum Oceania, South-east Asia, Latin America Papua New Guinea study autosomal recessive gene M. ferrugineum China, Korea, Japan Clinical disease Tinea barbae Tinea capitis Tinea corporis Tinea cruris Tinea favosa Tinea incognito Tinea imbricata Tinea manuum Tinea pedis Tinea unguium Onychomycosis T. rubrum M. canis T. tonsurans T. violaceum T. rubrum M. gypseum T. mentagrophytes T. rubrum E. floccosum T. schoenleinii ???? T. concentricum T. rubrum T. rubrum T. interdigitale E. floccosum T. rubrum T. interdigitale E. floccosum Diagnosis Microscopy Skin & Nails septate hyphae +/- arthroconidia Hair roots – spores +/- hyphae ectothrix endothrix Culture Surface colour - white, buff, olive, yellow Reverse – red, brown, yellow or none Texture – granular, suede, downy Topography – raised, furrowed, flat Slow or rapid growth Pathogenic dermatophytes grow at 35 C Microscopy - culture Microscopy cellotape preparation or tease mount Presence of: Microconidia pyriform, clavate, spherical/subspherical Microscopy - culture Macroconidia club, clavate, cigar, spindle, ellipsoidal, thick/thin/rough walled, septate Chlamydoconidia Spiral hyphae Identification media Dermatophytes Lactrimel Agar Pigment & conidia Trichophyton Sabouraud plus 5% NaCl Urea Agar (Christensen‟s Urea Agar) Trichophyton Agars 1-7 Peptone Agar Microsporum Rice Grain Agar Trichophyton rubrum Onychomycosis Growth 1 week Downy, rare granular - red brown reverse Clavate or pyriform microconidia “Barbed-wire” Trichophyton interdigitale Tinea pedis Growth <1 week - granular suede or downy Yellow-brown or no reverse colour Subspherical or clavate microconidia +/- spirals Epidermophyton floccosum Tinea cruris Growth 1 week – colony, khaki coloured No microconidia Large smooth walled macroconidia Numerous chlamydoconidia in chains Trichophyton tonsurans Tinea capitis - endothrix Growth 1 week Yellow-brown suede to granular colony Reverse yellow brown or red-brown Variable clavate & pyriform microconidia “balloon microconidia” chlamydoconidia present Trichophyton mentagrophytes Patient: 11yr old male Scalp lesion swabbed White to cream granular colony 2-3 days Numerous round microconidia - Spirals Kerion - guinea pigs Microsporum gypseum Patient: 3yr old male Scalp lesion swabbed Buff granular colony 2-3 days Broad ellipsoidal (2-6 celled) macroconidia Kerion – soil, „insect bites‟ - „dirty fingernails‟ Microsporum canis Patient: 7yr old female Scalp lesion swabbed Flat white mycelial growth 2-3 days Yellow reverse Spindle shaped macroconidia - terminal knob Kerion – kittens and kids Rare isolates Trichophyton verrucosum Inflammatory lesion - cattle ringworm Trichophyton violaceum Endothrix hair infection – „black dot‟ tinea capitis Trichophyton soudanense Anthropophilic – tinea capitis – Africa Microsporum audouinii Anthropophilic – ectothrix - tinea capitis Images from Kaminski‟s Digital Image Library of Medical Mycology and SNP Image Library l.hafner@qut.edu.au ann_mcphee@snp.com.au Yeast Identification Louise Hafner Ann McPhee Yeasts Unicellular fungal organism reproduce by budding - blastoconidia Not inherently pathogenic - alteration of host‟s cellular defences physiology normal flora Candida, Cryptococcus, Malassezia Trichosporon, Geotrichum, Rhodotorula Ref: Atlas of Clinical Fungi – GS de Hoog et al Candidiasis Candida albicans – most common isolate Candida species C. tropicalis C. parapsilosis C. guillermondii C. glabrata C. krusei C. lusitaniae C. kefyr C. dubliniensis All ubiquitous occur naturally on humans skin, buccal & vaginal mucosa, GI tract Candida - microscopy Scrapings, Swabs, Sputum, Urine, Pus, Blood CFW- KOH Gram stain Yeast cells +/- pseudohyphae Candida - culture Clinical specimen Sabourauds + antibiotics Sabourauds + antibiotics + cycloheximide C. albicans grows Cryptococcus does not Other yeasts variable growth 28/35 C Aerobic 2 - 3 days Quantitate growth – normal flora Candida - identification Germ tube production inoculate serum - incubate 2 hrs (<4hrs) 37 C Germ tubes parallel sides-no septa-no constriction C. albicans +ve C. dubliniensis +ve Identification - CAC ChromAgar Candida 48hrs 35ºC C. albicans blue-green C. tropicalis steel blue C. krusei matt pink C. dubliniensis forest green C. glabrata purple pink Refer to manufacturer‟s colour chart Further identification Carbohydrate assimilation commercial kits - API 20C AUX automated – Vitek YST Morphology & chlamydoconidia CMA & coverslip - Dalmau plate 28 C & examine 2-3 days C. albicans - chlamydoconidia BSA (GACA) C. dubliniensis chlamydoconidia and „feet‟ Cryptococcosis Cryptococcus neoformans C. neoformans var. grubii C. neoformans var. neoformans Europe, North America Woody debris “pigeons” Cryptococcosis Cryptococcus gattii C. neoformans var. gattii Eucalyptus spp. - E. camaldulensis Africa, East Asia, Australia, California Douglas fir tree Vancouver Island Canada A B C D Worldwide Subtropical Tropical Subtropical Tropical Europe Woody debris “Pigeons” River red gums E. camaldulensis Woody debris Almond (Colombia) Woody debris “Pigeons” var grubii (var neoformans) var gattii var gattii var neoformans AIDS Immune deficient Healthy Non-AIDS disease Healthy Non-AIDS disease AIDS Immune deficient BSA (GACA) Brown BSA (GACA) Brown BSA (GACA) Brown BSA (GACA) Brown CANA(CGB) -ve CANA(CGB) +ve (Blue) CANA(CGB) +ve (Blue) CANA(CGB) -ve Unknown Filobasidiella bacillispora Filobasidiella bacillispora Filobasidiella neoformans Ref: Atlas of Clinical Fungi – GS de Hoog et al Filobasidiella teleomorph Clamped hyphae & basidia Basidiospores - 1.8-3m Convert to yeast cells Basidiospores - infective particle Size allows alveolar deposition F. neoformans - C. neoformans spherical spores F.bacillispora - C. gattii reniform spores Cryptococcus - microscopy Spherical/ellipsoidal budding yeast (3.5-7.5x3-7m) Pseudohyphae rare Capsules +/- Germ Tube –ve Urease +ve Basidiomycetous yeast Teleomorph clamp connections Cryptococcus - culture Mucoid colony on all media Brown colonies on Birdseed Agar 2-3 days Vitek yeast ID 99% C. neoformans CGB - Canavanine Glycine Bromothymol blue agar C. neoformans/grubii – no growth/no colour change C. gattii - blue Trichosporon sp. (beigelii) T. asahii systemic infections T. asteroides superficial skin infections T. cutaneum superficial skin infections T. inkin pubic white piedra T. mucoides systemic infections T. ovoides white piedra scalp Trichosporon sp. White piedra, nail infections Basidiomycete Oval budding yeast cells Arthroconidia Urea positive Raised wrinkled colony CAC - blue Geotrichum sp. Rare infections – pulmonary, cutaneous Ascomycete Arthroconidia, true hyphae & few yeast cells Urea negative Flat spreading colony with aerial mycelia CAC – pale pink Malassezia sp. M. furfur Human flora - pityriasis M. pachydermatis Animals – dogs not lipophilic M. sympoidialis Human – normal flora M. globosa Human – normal flora pityriasis M.obtusa Human – normal flora atopic dermatitis M.restricta Human – normal flora M.slooffiae Human /pig - flora Yeast identification Positive germ tube – C. albicans Consider C.dubliniensis Encapsulated yeast – speciate Isolates from sterile sites – speciate Full identification – isolates from immunosuppressed patients chronic infections recalcitrant infections Antifungal therapy Antifungal susceptibility testing - Vitek AST-YS01 – AmB, Flu, 5-FC, Vori - Sensititre YeastOne - Reference Laboratory Speciation - access to antifungal susceptibility profiles - provides a guide to therapy www.mycology.adelaide.edu.au „Antifungal Susceptibility Testing‟ Biofilm production by clinical isolates of Candida spp. C.P Girish Kumar &Thangam Menon Medical Mycology Feb2006,44,99-101 Biofilms are universal, complex, interdependent communities of surface-associated microorganisms, enclosed in an exopolysaccharide matrix occurring on any surface, including medical devices. …..biofilms are notoriously difficult to eliminate …infections caused...urinary tract, catheter, child middle-ear, dental plaque, to more threatening infections, such as endocarditis and infections of heart valves. Microbial biofilms serve as a nidus for disease… are often associated with high-level antimicrobial resistance Biofilms Medical devices – heart valves, catheters, optical lens, surgical prostheses & screws Scrape surface Embed pieces of device in culture media Vortex may not loosen biofilm Antifungal susceptibilities of isolates may not equate with „in vivo‟ status www.erc.montana.edu Other Yeast-like isolates Sporothrix schenckii (dimorphic) Yeast cells 2-6µm - Narrow based buds „Cigar bodies‟ (Histology asteroid body) Histoplasma capsulatum (dimorphic) Yeast cells 2-4µm - Narrow based buds In macrophages (Histology sections) Other Yeast-like isolates Pneumocystis jirovecii (carinii) DFA /Silver stain Non budding, round, ovoid, collapsed crescent forms (2-5µm dia) in small clusters Prototheca sp. (Achloric algae) Sporangia (2-26µm dia) No budding or hyphae Rare Yeast-like - travellers Blastomyces dermatitidis Thick walled, broad based bud (8-14µm) Coccidioides immitis/posadasii Spherules (10-100µm) & endospores (2-5µm) No buds Paracoccidioides brasiliensis Round/oval (3-30µm), multiple budding Penicillium marneffei Round/oval (3µm), septate, no buds In macrophages Images from Kaminski‟s Digital Image Library of Medical Mycology and SNP Image Library l.hafner@qut.edu.au ann_mcphee@snp.com.au Wot R U? Louise Hafner Ann McPhee Arxiella terrestris and next… Talk to Reference Laboratory Send isolate for identification Speak to clinician Not identified yet but not… Fusarium, Paecilomyces, Acremonium, Scedosporium, Aspergillus, Curvularia May help to direct antifungal therapy Mould Identification Emergence of opportunistic fungal pathogen Then Now Opportunistic fungi Cosmopolitan fungi Low virulence Diverse range causative organisms Debilitated host Altered immunity New medical procedures Zygomycetes Tissues eg lung, rhinocerebral sinusitis Diabetes mellitis, immunosuppression Wide hyphae, thin walled, sparse septa Microscopy important, maybe no growth Treatment - Surgery, Amphotericin B Rhizopus, Mucor, Absidia Apophysomyces, Saksaenaea Growth temperatures - 30,35,40,55ºC Zygomycetes Rhizopus oryzae Zygomycetes - identification Ref: Atlas of Clinical Fungi – GS de Hoog et al Hyphomycetes - identification Colony description Surface texture cottony, velvet, granular, glabrous Topography flat, raised. domed, radial grooves Surface & reverse pigments white, yellow, brown, green, grey etc. Growth rate depends on media & temperature Microscopy of culture Conidia Structures producing conidia Hyalohyphomycetes Hyalohyphomyctes Hyphae colourless Conidia production - colour Aspergillosis Common clinical isolates: Aspergillus fumigatus Aspergillus flavus Aspergillus niger Aspergillus terreus Aspergillus nidulans A. fumigatus A.niger Aspergillosis Paranasal sinusitis Pulmonary disease - Sputum, Bronchial washings Disseminated disease - Tissue biopsy, Blood cultures Septate hyphae with 45º branching Gram, CFW (Calcofluor White) GMS (Grocott‟s Methenamine Silver), PAS (Periodic acid-Schiff) Aspergillus - Identification Macroscopic – colour & topography Microscopic appearance vesicle shape – flask or globose phialides – uniseriate, biseriate, columnar, radiate conidia -round/oval rough(echinulate) smooth hyaline pigmented Pseudallescheriasis Scedosporium infection Disease spectrum similar to Aspergillus Mycetoma, Lung infection Paranasal sinusitis, Mycotic keratitis Otitis externa, Mastoiditis Traumatic injury can implant fungi Immunocompromised - rapid dissemination Causative fungi Scedosporium apiospermum Pseudallescheria boydii – teleomorph Teleomorph not usual clinical isolate Scedosporium prolificans (S. inflatum) Australia & Spain Soil & water borne saprophytes Direct microscopy – septate hyphae Culture – rapid growth Scedosporium aurantiacum New species Described Gilgado et al. 2005 Conidiogenous cells & conidia similar to S. apiospermum Light yellow diffusible pigment PDA Optimum growth temp. 37- 40 C, max. 45 C Best distinguished by genetic analysis S.prolificans S.apiospermum Growth--rapid flat Grey then black Growth—rapid fluffy White then grey Oval conidia Basally swollen Flask shaped annelides Oval conidia Elongate conidiophores Ring-like annelations Multiple resistance Antifungals Variable resistance Antifungals Sensitive cycloheximide Resistant cycloheximide Teleomorph unknown Pseudallescheria boydii Graphium unknown Graphium synanamorph Phialidic conidia production Collection Corneal Ulcer Culture Fusarium solani Patient: Male 53 yrs Corneal ulcer - Gram stain: hyphae seen Fungus grew 3 days – one colony PDA & Slide cultures on Cornmeal Agar Microconidia, fusiform macroconidia & chlamydoconidia Paecilomyces lilacinus Growth rapid, floccose, violet Pigmented conidiophore ?rough walled Phialides swollen base taper to thin neck (penicillate) Conidia ellipsoidal (2.5x2.0µm) roughened or smooth divergent chains Growth 35°C & Cycloheximide Paecilomyces spp. environmental moulds Slender hyaline septate hyphae Single-celled (ameroconidia) microconidia Paecilomyces Acremonium Fusarium All colours/Floccose Not blue-green Rapid White/Coloured Moist/Powdery Slow White/Brightly Coloured/Fluffy Rapid Phialides tapered Conidia in chains Conidia in slimy heads Large fusiform macroconidia Hyphomycetes Phaeohyphomycetes (Dematiaceous) Hyphae pigmented – yellow to brown/black - melanin Conidia pigmented Culture – brown/grey/black Conidia produced on PDA (Potato Dextrose Agar) Conidia phragmo didymo amero dictyo ‘Jackson 5’… ABC Easy As 123 In Mycology… Easy As ABCDE ABCDE of Mycotic keratitis, Paranasal sinusitis and Phaeohyphomycosis • • • • Rapid growth Fluffy, grey-brown-black colony Saprophytes of soil and vegetation Dematiaceous fungus - phaeohyphomycete Multi celled conidia Dictyoconidia Obpyriform with beaks Pseudoseptate conidia on zig zag rachis Alternaria Bipolaris 3 + transverse septa curved with central cell larger & darker Phragmoconidia Taxonomically referred to Bipolaris & Exserohilum Conidia multiseptate protruding hilum septum above hilum thickened & dark Curvularia Drechslera Exserohilum Other isolates - Basidiomycetes Nasal sinus material Culture grew 3 days 25ºC White, cottony, malodourous Clamp connections LCB (Lactophenol Cotton Blue) Basidiomycete – mushroom Schizophyllum commune Others - Coelomycetes ostiole Conidia in fruit bodies Phialides line inner wall Propagules mitotic, non-sexual Pycnidium Fruit bodies spherical - pycnidia Open cup shaped - acervuli Modified Sachs‟ with maize leaf Acervulus Ascomycetes - teleomorph Cleistothecium enclosed ascocarp with dispersed asci Perithecium enclosed ascocarp with apical ostiole asci in basal tuft Coelomycetes Plant pathogens “black spot” crops & fruit Tropical & subtropical climates Clinical disease – environmental trauma − Subcutaneous hyphomycosis − Keratitis Subcutaneous mycoses „the inoculation mycoses” Chronic & localized Traumatic implantation Soil saprophyte – regional epidemiology Adapted to tissue environment thermal dimorphism tissue dimorphism resistant microcolonies Disease Sporotrichosis Chromoblastomycosis Mycetoma Phaeohyphomycosis Zygomycosis Mucormycosis Entomophthoromycosis Rhinosporidiosis Lobomycosis Sporothrix schenckii Cladophialophora Fonsecaea, Phialophora Pseudallescheria, Maduralla Acremonium, Exophiala Exophiala, Bipolaris Rhizopus, Rhizomucor, Absidia Saksenaea, Apophysomyces Basidiobolus, Conidiobolus Rhinosporidium seeberi Loboa loboi Diagnosis Tissue biopsy & rarely skin scrapings Microscopy PAS & GMS CFW Histopathology – best microscopic diagnosis Communication histology & microbiology Culture Sabourauds & BHI Slow growing 1-2 weeks Dematiaceous fungi Clinicopathologic & mycologic definitions Mycetoma Chromoblastomycosis Phaeohyphomycosis Grain Sclerotic body Mycelial budding Medium range Small range Wide range Discharging sinuses Marked hyperplasia Subcutaneous inflammation Heterogenous Cystic and other Exophiala jeanselmei complex Patient: Female 90yrs Pustule over thumb joint swabbed Gram stain: Yeast cells & hyphae Blood Agar 72hrs Small black yeast-like colonies Exophiala jeanselmei complex Velvety texture with aerial hyphae Tapered annelides Conidia form on lateral pegs Cluster in globes Why identify fungi? Common in nature - Rare in human infections Identification gives access to antifungal profiles www.mycology.adelaide.edu.au May not affect treatment eg surgical debridement Experience useful when disease is present Access to literature & treatment regimes Collate results from reference laboratory – good resource for advice on treatment Fun for scientists References Atlas of Clinical Fungi G.S. de Hoog, J. Guarro et al, Centraalbureau voor Schimmelcultures, Utrecht Netherlands 2000 Descriptions of Medical Fungi David Ellis, Stephen Davis, Helen Alexiou, Rosemary Handke, Robyn Bartley. Second Edition 2007 Genera of Hyphomycetes JW Carmichael, WB Kendrick et al. The University of Alberta Press. First Edition. Canada. 1980 Illustrated Genera of Imperfect Fungi H.L. Barnett & Barry B. Hunter. Fourth Edition Medically Important Fungi A Guide to Identification Davise H. Larone ASM Press 2002 Medical Mycology – A Self-Instructional Text Kern, M.E F.A. Davis Company. USA. 1988 Mycology Workshop ICM8 2006 Summerbell R. www.mycology.adelaide.edu.au Images from Kaminski‟s Digital Image Library of Medical Mycology and SNP Image Library l.hafner@qut.edu.au ann_mcphee@snp.com.au Graduation