Worksheet 2 – Chapter 14 – Chemical Kinetics 1. The rate equation
advertisement
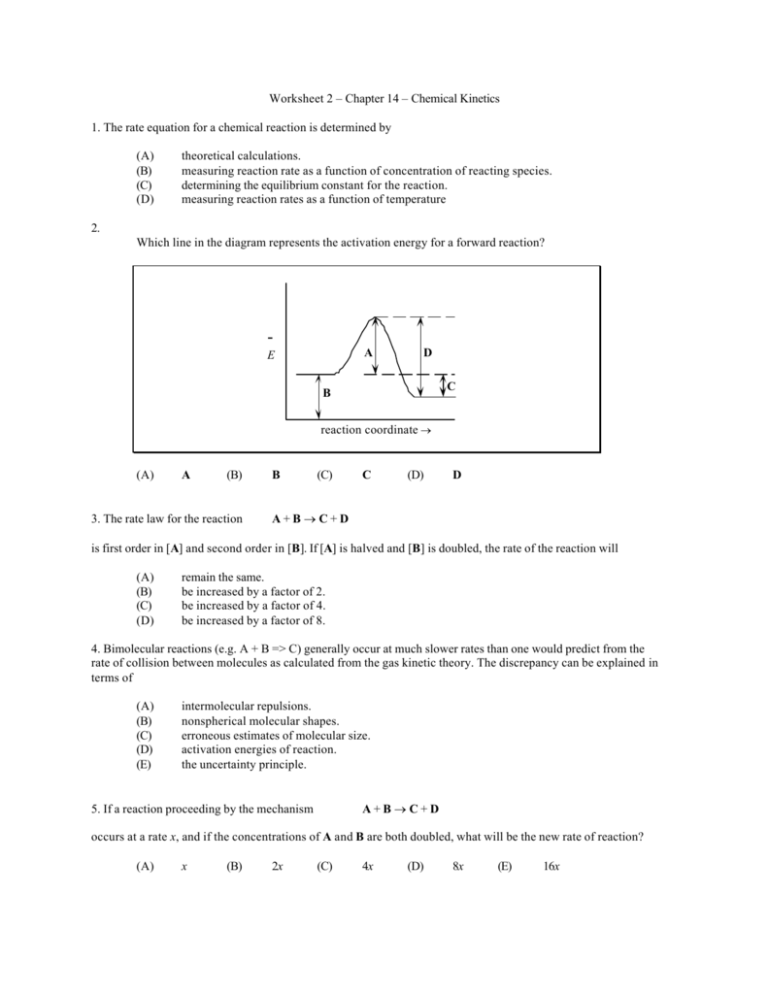
Worksheet 2 – Chapter 14 – Chemical Kinetics 1. The rate equation for a chemical reaction is determined by (A) (B) (C) (D) theoretical calculations. measuring reaction rate as a function of concentration of reacting species. determining the equilibrium constant for the reaction. measuring reaction rates as a function of temperature 2. Which line in the diagram represents the activation energy for a forward reaction? A E D C B reaction coordinate → (A) A (B) 3. The rate law for the reaction B (C) C (D) D A+B→C+D is first order in [A] and second order in [B]. If [A] is halved and [B] is doubled, the rate of the reaction will (A) (B) (C) (D) remain the same. be increased by a factor of 2. be increased by a factor of 4. be increased by a factor of 8. 4. Bimolecular reactions (e.g. A + B => C) generally occur at much slower rates than one would predict from the rate of collision between molecules as calculated from the gas kinetic theory. The discrepancy can be explained in terms of (A) (B) (C) (D) (E) intermolecular repulsions. nonspherical molecular shapes. erroneous estimates of molecular size. activation energies of reaction. the uncertainty principle. A+B→C+D 5. If a reaction proceeding by the mechanism occurs at a rate x, and if the concentrations of A and B are both doubled, what will be the new rate of reaction? (A) x (B) 2x (C) 4x (D) 8x (E) 16x 6. Given that 1 → ← 2SO3(g) 2 The forward reaction (1) is proceeding at a certain rate at some temperature and pressure; when the pressure is increased, we may expect for the forward reaction (1) 2SO2(g) +O2(g) (A) (B) (C) (D) (F) a greater rate of reaction and a greater yield of SO3 at equilibrium. a greater rate of reaction and the same yield of SO3 at equilibrium. a lesser rate of reaction and a lesser yield of SO3 at equilibrium. a lesser rate of reaction and a greater yield of SO3 at equilibrium. no change in rate or yield. 7. The addition of a catalyst in a chemical reaction (A) (B) (C) (D) increases the concentration of products at equilibrium. increases the fraction of reactant molecules with a given kinetic energy. provides an alternate path with a different activation energy. lowers the enthalpy change in the overall reaction. 8. The oxidation of ammonia produces nitrogen and water via the reaction 4NH3 + 3O2 → 2N2 + 6H2 O. If the rate of formation of N2 is 2.0 mol/(L • s), then the rate at which a. H2 O is being formed is 2.0 mol/(L • s). b. NH3 reacts is 4.0 mol/(L • s). c. O2 reacts is 1.5 mol/(L • s). d. NH3 reacts is 0.50 mol/(L • s). e. H2 O is being formed is 0.67 mol/(L • s). 9. From a consideration of the following reaction system 2H2 S(g) + O2 (g) → 2S(s) + 2H2 O(g) we can conclude that a. the reaction is second order in H2 S and first order in O2 . b. the reaction is first order in H2 S and second order in O2 . c. rate = k[H2 S]2 [O2 ]. d. rate = k[H2 S][O2 ]. e. None of these conclusions are justified. 10. Nitrosyl chloride is produced from the reaction of nitrogen(II) oxide and chlorine. 2NO(g) + Cl2 (g) → 2NOCl(g) The following initial rates at a given temperature were obtained for the concentrations listed below: Rate Experiment 1 2 3 mol L • hr 2.21 8.83 17.5 NO mol L mol Cl 2 L 0.25 0.50 0.50 0.25 0.25 0.50 The experimental rate law is a. rate = k[NO]. b. rate = k[NO]1/2 [Cl2 ]. c. rate = k[NO][Cl2 ]1/2 . d. rate = k[NO]2 [Cl2 ]. e. rate = k[NO][Cl2 ]. 11. The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the equation OCl- + I- → OI- + Cl- The rate of formation of OI- is given by the rate law Rate = k [I- ][OCl- ] [OH- ] The overall reaction order and the order with respect to OH- are a. 0 and 1. b. 0 and –1. c. 1 and –1. d. 2 and 1. e. 2 and –1. 12. The following mechanism has been proposed for the formation of ethylbenzene: CH3CH2Br + AlBr3 → AlBr4– + CH3CH2+ CH3CH2+ + C6H6 → C6H6CH2CH3+ C6H6CH2CH3 + AlBr4– → AlBr3 + HBr + C6H5CH2CH3 Which substance serves as the catalyst? (A) (B) AlBr3 AlBr4– (C) (D) CH3CH2+ C6H6CH2CH3+ 13. The table presents data for the reaction: 2H2(g) + 2NO(g) k1 → 2H2O(g) + N2(g) The temperature of the reaction is constant. The initial rate is in arbitrary units. Exp. I II III IV Initial Concentration (mol·L–1 ) [NO] × 10– 3 [H 2] × 10– 3 6.0 1.0 6.0 2.0 1.0 6.0 2.0 6.0 Initial Rate 18 36 3 12 What is the rate law for this reaction? (A) (B) rate = k 1 [H2] [NO] rate = k 1 [H2]2 [NO]2 (C) (D) rate = k 1 [H2]2 [NO] rate = k 1 [H2] [NO]2 14. The reaction 2A + 2B → C + D proceeds by this mechanism: 2A → ← A2 A2 + B → X + C X+B →D (equilibrium) (rate determining) (rapid) The rate equation for the reaction is (A) rate = k[A] [B] (C) (B) rate = k[A]2 [B]2 (D) [Al2 [B]2 [C] [D] rate = k[A]2 [B] rate = k 15. For the reaction X2 + Y + Z → XY + XZ the mechanism was determined to be l) X2 + Y → XY + X 2) X + Z → XZ (very slow) (very fast) What is the rate law for this reaction? (A) rate = k [X2] [Y] [Z] (C) rate = k [X] [Z] (B) rate = k [X2] (D) rate = k [X2] [Y] 16. In aqueous solution, iodine reacts with acetone as represented by the following stoichiometric equation: I2 + CH3 COCH3 → CH3 COCH2 I + H+ + I- The experimental rate law is Rate = k[H+][CH3 COCH3 ] According to the information above, an increase in the hydrogen ion concentration has what effect on the reaction? a. It increases the value of the equilibrium constant. b. It decreases the value of the equilibrium constant. c. It increases the rate of the reaction. d. It decreases the rate of the reaction. e. It does not affect the rate of the reaction. 17. For the reaction 6CH2 O + 4NH3 → (CH2 )6 N4 + 6H2 O the rate is expressed as a. 1 ∆[ H 2 O] . An equivalent expression is 6 ∆t 1 ∆[(CH 2 ) 6 N 4 ] . 2 ∆t ∆[CH 2 O] . ∆t b. 6 c. –6 d. – 1 ∆[NH3 ] . 4 ∆t e. – 1 ∆[H 2 O] . 6 ∆t ∆[CH 2 O] . ∆t 18. The gas-phase decomposition of N2 O5 is a first-order process with a rate constant of 1.50 x 10-3 /s at 55°C. The decomposition reaction is N2 O5 (g) → 2NO2 (g) + 12 O2 (g) Ten (10.0) g of N2 O5 are placed in vessel 1 and 5.0 g of N2 O5 in vessel 2. The vessels are at the same temperature and pressure. How much time is required for half of the N2 O5 to decompose in each vessel? a. Vessel 1 requires twice as much time as vessel 2. b. Vessel 2 requires twice as much time as vessel 1. c. Vessel 1 requires three times as much time as vessel 2. d. Vessel 1 requires four times as much time as vessel 2. e. Vessel 1 requires the same amount of time as vessel 2. • Exam 1 – Wednesday, September 18 – 12:30 PM – Renfrew 111 • Chapters 11 – 12 – 14 • Friday’s lecture will begin Chapter 15, bring lecture notes to class. 1.B 2.A 3.B 4.D 6. A 7.D 8.b 9.e 10.d 11.c 12.A 13.D 14.D 15.D 16.c 17.d 18.e 5.C









![CHEM 1520 SI MON, TUES, & WEDNES 1.Calculate [H3O+] in a](http://s3.studylib.net/store/data/007346334_1-b78d73402f58153c92290299886ff084-300x300.png)