CHEM-303 (Chapter 7 IR)
advertisement

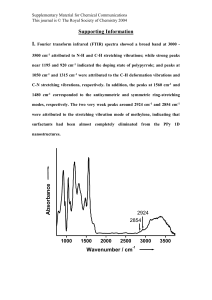
Chapter 7 Infrared Spectrometry Spectroscopy is the study of the interaction between radiant energy and matter. Energy applied to matter can be absorbed, emitted, cause a chemical change, or be transmitted. The wavelengths at which an organic compound absorbs radiant energy are dependent upon the structure of the compound. Spectroscopic techniques are used to determine the structures of unknown compounds. 1 Electromagnetic Radiation Electromagnetic radiation is the energy that is transmitted through space in the form of waves. 2 Each type of electromagnetic radiation is characterized by its wavelength (λ) Wavelength (λ) is the distance from the crest of one wave to the crest of the next wave. 3 Radiation may also be characterized by its frequency (ν) which is the number of complete cycles per second (cps), also called Hertz (Hz). In IR, frequency is expressed as wavenumbers: the number of cycles per centimeter Wavenumbers have units of reciprocal centimeters (cm-1) 4 The relationship between wavelength (or frequency) and energy (E) is well defined Wavelength and frequency are inversely proportional . The higher the frequency, the greater the energy of the wave. The shorter the wavelength, the greater the energy of the wave. ultraviolet 5 visible infrared radio increasing λ (or decreasing ν) means decreased energy Types of Energy Transitions in Each Region of the Electromagnetic Spectrum REGION ENERGY TRANSITIONS X-ray Bond-breaking UV/Visible Electronic Infrared Vibrational Microwave Rotational Radio Frequency Nuclear and Electronic Spin (NMR) 6 Features of a Spectrum When a sample absorbs photons from a beam of transmitted radiation, the intensity of the transmitted radiation decreases. ¾ It is this change in intensity that is measured in absorption spectroscopy. An infrared spectrum of a compound is a plot of percent transmission (%T) versus either wavelength or frequency changing. 7 %T = (Intensity/original intensity) x 100 Absorption of Infrared Radiation IR spectroscopy is a method for functional group analysis. Nuclei of atoms bonded by covalent bonds undergo vibrations or oscillations. When molecules absorb IR radiation, the absorbed energy causes an increase in the amplitude of the vibrations of the bonded atoms. Different type of bonds ( C-H, C-C, C-O, C=O, O-H) absorb IR radiation at different λ. A bond within a molecule may absorb energy at more than one wavelength. O bending R O H R H at 3330 cm-1 stretching 1250 cm-1 The relative amount of absorbed energy depends on the change of bond moment: Non-polar bonds (e.g., C-C, C-H bonds) Î weak absorption Polar bonds (e.g., O-H, C-O, C=O bonds) Î strong absorption 8 9 INFRARED SPECTROSCOPY * * * * * ν = frequency of absorption c = speed of light k = force constant m 1m m* = reduced mass = k m* υ= 1 2π c 2.5 µ 3 m* for: CH = 12/13 =0.92 CD = 24/14 =1.7 CC =144/24 = 6.0 CCl = 450/49.5 = 9.1::::725 cm-1 2 m 1 +m 2 4 5 6 7 C C H C 3100 H H2N 2850 2750 C C C C 9 8 10 -1 460 N.m 1090 1630 12 C H N H O H 15 O C N 1630 - 1690 2200 3300 Aromatic, C=C 1450 - 1600 ~3200 - 3700 (4 peaks) H O H C O 2900 20 C C (w) C O C N X 1000 - 1200 H O C 680 - 900 C O 3000 3500 2500 2000 -1 cm Frequency Wavenumber %T = 10 25 For alkenes: (=CH; =CH2 bending) 690-990 weak Y 1640 - 1820 4000 580 N.m-1 710 840 shoulder (sh) 1600 - 1700 D C C C C N C H C HN H O k for C C more polar the bond - stronger the peak HN %Transmittance * * * * * ,υ = C λ ,υ = cps or Hz 1 1 = × 10 λ cm λ µ Intensity Original Intensity ×100 = 4 cm I ×100 IO −1 1800 1600 1400 1200 1000 800 600 400 Fingerprint region ketone 1715 aldehyde 1725 ester 1735 (CH bending) X acid: dimer 1710 monosubstituted: ~700; ~750 monomer 1760 (two peaks) Y disubstituted: amide: amide I band 1690-1630 (C=O) ortho: ~750 (one peak) amide II band 1515-1670 (NH) para: ~830 (one peak) acid halide, RCOCl 1785-1815 meta: ~700; 780; 880 acid anhydride 1740-1840 (usually two peaks) (three peaks) carboxylate 1590-1600 For CHEM 202, 303 & higher level courses ..........Designed, drawn and compiled by Prof SA ALI Preparation of the sample Compounds that are subjected to IR analysis should be pure. Solids should be samples from recrystallization or from chromatography. Liquids should be samples from distillation or from gas chromatographic separation. Liquids can be examined as a thin film (neat) between two salts (KBr) plates or can be dissolved in a nonpolar organic solvent (e.g., CCl4). FTIR instruments can subtract the solvent spectrum from the spectrum of a sample in that solvent. For solid compounds, make pellet with KBr powder (1 mg solid to 100 mg KBr). Using only dry equipment and dry chemicals will minimize the appearance of water at 3000-3800 cm-1 (O-H stretching) and 1520-1750 cm-1 (O-H bending). 11 The Infrared Spectrum The instrument used to measure absorption of infrared radiation is called infrared spectrophotometer 12 Interpretation of IR Spectrum The region from 1400-4000 cm-1 (absorption from stretching) is especially useful for identification of the various functional groups. The region to the right of 1400 cm-1 is often quite complex (absorption from stretching and bending): fingerprint region. 13 14 A. Carbon-Carbon and Carbon-Hydrogen Bonds C-C single bonds give rise to weak absorption bands in the IR spectrum: not very useful for structure determination. Almost all organic compounds contain C-H bonds The C-H stretching peaks are often useful in determining the hybridization of the carbon atom. 15 Alkane (octane) 16 ALKANE Hexane bending CH2 bend CH3 bend CH stretch CH3 CH2 CH2 CH2 CH2 CH3 17 stretching stretching 18 bending Cyclohexene 1648 cm-1 720 cm-1 19 ALKYNE 1-Hexyne C≡C CH2, CH3 ≡C-H 20 C-H HC Symmetrical alkynes do not show a peak at this range. C CH2 CH2 CH2 CH3 1-Heptyne 2120 cm-1 21 NITRILE Propanenitrile = C=N CH3 CH2 C 22 N B. Substituted Benzenes _ The aryl C_H stretching gives weak peaks near 3030 cm -1 _ The aryl C_C stretching gives four peaks between 1450-1600 cm -1 -1 stretching at ~3030 cm (weak) 1450-1600 cm-1 (four peaks) H Y The C-H Bending Absorption of Substituted Benzenes cm-1 is as follows: monosubstituted two peaks: 730-770 and 690-710 cm-1 ortho-disubstituted one peak: 735-770 cm-1 para-disubstituted one peak: 790-840 cm-1 meta-disubstituted three peaks: 860-900, 750-810, and 680-730 cm-1 23 Substituted benzene rings have distinctive patterns at 1667-2000 cm-1 These bands are due to overtones of aromatic bands. Such use of this region to identify substitution patterns can be difficult since these bands are weak, and strong bands, such as the carbonyl bands, can obscure the overtone patterns. 24 Toluene monosubstituted C-H Bending two peaks : 730-770 and 690-710 cm-1 The aryl C_C stretching: four peaks between 1450-1600 cm -1 25 26 chlorobenzene monosubstituted C-H Bending two peaks : 730-770 and 690-710 cm-1 The aryl C_C stretching: four peaks between 1450-1600 cm -1 27 o-chlorotoluene ortho-disubstituted one peak: 735-770 cm-1 The aryl C_C stretching: four peaks between 1450-1600 cm -1 28 m-chlorotoluene meta-disubstituted three peaks: 860-900, 750-810, and 680-730 cm-1 The aryl C_C stretching: four peaks between 1450-1600 cm -1 29 p-chlorotoluene para-disubstituted 30 one peak: 790-840 cm-1 The aryl C_C stretching: four peaks between 1450-1600 cm -1 ¾ Nitrobenzene Nitro group shows a coupled pair of IR bands at 1343 and 1519 cm-1 in the IR spectrum. The C-N stretching for the nitroaromatic is at 860 cm-1 31 C. Haloalkanes: C-X Î 500-1430cm-1 (fingerprint region) 32 Carbon Tetrachloride Cl Cl C Cl C-Cl Cl 33 Often used as a solvent for IR spectra. When it is used, spectra show C-Cl absorptions. D. Alcohols and Amines OH or NH: 3000-3700 cm-1 (strong for OH; medium or weak for NH) C_O or C_N: 900-1300 cm-1 (fingerprint region) Hydrogen bonding changes the position and appearance of an infrared absorption band. 34 35 36 1o Amines RNH2 3000-3600cm-1(medium or weak double peaks) + C-N (900-1300 cm-1) 37 2o Amines R2NH 3000-3600cm-1(medium or weak one peak) + C-N (900-1300 cm-1) 38 3o Amines R3N No C-H peak - Only C-N peak at 900-1300 cm-1 39 40 41 E. Ethers 42 Ether have C-O stretching band that falls in the fingerprint region at 1050-1260 cm-1 Because oxygen is electronegative, the stretching causes a large change in bond moment, therefore C-O absorption is usually strong. Alcohols, esters, and other compounds containing C-O single bonds also show absorption here. Anisole AROMATIC ETHER C-H aromatic O CH3 benzene C-O 43 F. Carbonyl Compounds Generally the carbonyl group gives a strong peak which occurs at 1630-1780 cm-1 The exact location depends on the actual functional group present, ring strain, conjugation, and other substituents. 44 Ketones: C=O Î 1680-1750 cm-1 (strong) 45 46 Aldehydes: Aldehyde carbonyl (C=O) shows strong absorption at 1720-1740 cm-1 Also, the aldehyde C-H bond shows two characteristic stretching bands (just to the right of the aliphatic CH band) at 2820-2900 cm-1 and 2700-2780 cm-1 Both these peaks are sharp, but weak, and the peak at 2900 cm-1 may be obscured by overlapping absorption of other CH bonds. 47 ALDEHYDE Nonanal CHO CH bend C=O O CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 C 48 H Carboxylic Acid: Exhibit typical carbonyl (C=O) absorption at1700-1725 cm-1 (strong) Also shows O-H stretching Î very broad from 3330- 2500 cm-1 Strong hydrogen bonding in the dimer weakens the OH bond and leads to a broad peak at lower frequency 49 Esters: The C=O band is observed at 1735-1760 cm-1 The C-O band is observed in the fingerprint region at 1100-1300 cm-1 50 Typical Infrared Absorption Regions (stretching vibrations) WAVELENGTH (µm) 2.5 4 O-H C-H 5 6.1 C=O C N N-H 5.5 C C X=C=Y Very few bands C=N C=C 6.5 15.4 C-Cl C-O C-N C-C (C,O,N,S) 4000 2500 2000 1800 1650 1550 FREQUENCY (cm-1) 51 650 ¾ Analysis of an IR Spectrum Look for major stretching and bending peaks. A very large peak in the 3200-3500 cm-1 range indicates the presence of an alcohol or phenol. Another strong peak is C=O stretching which is in the range of 1630-1850 cm-1 Moderate bands in the 3300-3500 cm-1 range could be indicative of N-H stretching. A peak around 2200 cm-1 could possibly be a nitrile or alkyne If a compound is aromatic, then aromatic peaks would appear in the NMR spectra. Multiple techniques can be used in identifying unknown compounds. 52 INFRARED SPECTROSCOPY * * * * * ν = frequency of absorption c = speed of light k = force constant m 1m m* = reduced mass = k m* υ= 1 2π c 2.5 µ 3 m* for: CH = 12/13 =0.92 CD = 24/14 =1.7 CC =144/24 = 6.0 CCl = 450/49.5 = 9.1::::725 cm-1 2 m 1 +m 2 4 5 6 7 C C H C 3100 H H2N 2850 2750 C C C C 9 8 10 -1 460 N.m 1090 1630 12 C H N H O H 15 O C N 1630 - 1690 2200 3300 Aromatic, C=C 1450 - 1600 ~3200 - 3700 (4 peaks) H O H C O 2900 20 C C (w) C O C N X 1000 - 1200 H O C 680 - 900 C O 3000 3500 2500 2000 -1 cm Frequency Wavenumber %T = 53 25 For alkenes: (=CH; =CH2 bending) 690-990 weak Y 1640 - 1820 4000 580 N.m-1 710 840 shoulder (sh) 1600 - 1700 D C C C C N C H C HN H O k for C C more polar the bond - stronger the peak HN %Transmittance * * * * * ,υ = C λ ,υ = cps or Hz 1 1 = × 10 λ cm λ µ Intensity Original Intensity ×100 = 4 cm I ×100 IO −1 1800 1600 1400 1200 1000 800 600 400 Fingerprint region ketone 1715 aldehyde 1725 ester 1735 (CH bending) X acid: dimer 1710 monosubstituted: ~700; ~750 monomer 1760 (two peaks) Y disubstituted: amide: amide I band 1690-1630 (C=O) ortho: ~750 (one peak) amide II band 1515-1670 (NH) para: ~830 (one peak) acid halide, RCOCl 1785-1815 meta: ~700; 780; 880 acid anhydride 1740-1840 (usually two peaks) (three peaks) carboxylate 1590-1600 For CHEM 202, 303 & higher level courses ..........Designed, drawn and compiled by Prof SA ALI END 54