SPM form 5 chemistry chap 4 exercises - E
advertisement
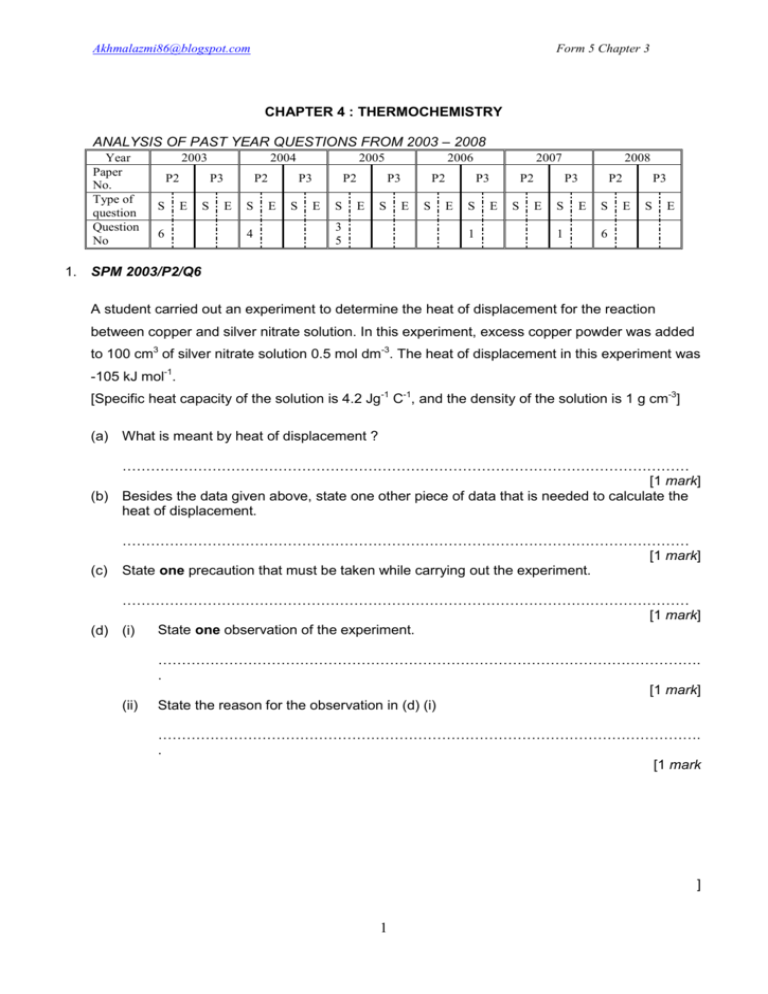
Akhmalazmi86@blogspot.com Form 5 Chapter 3 CHAPTER 4 : THERMOCHEMISTRY ANALYSIS OF PAST YEAR QUESTIONS FROM 2003 – 2008 Year Paper No. Type of question Question No 2003 P2 S 2004 P3 E 6 S P2 E S 4 2005 P3 E S P2 E S 2006 P3 E S 3 5 P2 E S 2007 P3 E S 1 P2 E S 2008 P3 E S 1 P2 E S P3 E S E 6 1. SPM 2003/P2/Q6 A student carried out an experiment to determine the heat of displacement for the reaction between copper and silver nitrate solution. In this experiment, excess copper powder was added to 100 cm3 of silver nitrate solution 0.5 mol dm-3. The heat of displacement in this experiment was -105 kJ mol-1. [Specific heat capacity of the solution is 4.2 Jg-1 C-1, and the density of the solution is 1 g cm-3] (a) What is meant by heat of displacement ? ………………………………………………………………………………………………………… [1 mark] (b) Besides the data given above, state one other piece of data that is needed to calculate the heat of displacement. (c) ………………………………………………………………………………………………………… [1 mark] State one precaution that must be taken while carrying out the experiment. ………………………………………………………………………………………………………… [1 mark] State one observation of the experiment. (d) (i) (ii) ……………………………………………………………………………………………………. . [1 mark] State the reason for the observation in (d) (i) ……………………………………………………………………………………………………. . [1 mark ] 1 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (e) Based on the information from this experiment, calculate (i) the number of moles of silver ions reacted. [1 mark] (ii) the amount of heat released. [1 mark] (iii) the change in temperature. [1 mark] (f) Draw an energy level diagram for the reaction in this experiment. [2 marks ] 2 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (g) The experiment is repeated using 100 cm3 of 1.0 mol dm-3 silver nitrate solution and excess copper powder. Calculate the temperature change in this experiment. Explain why this change of temperature is different from that in (e) (iii). ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… [3 marks] 2. SPM 2004/P2/Q4 Figure 4 show the set-up of the apparatus of an experiment to determine the heat of precipitation. 25.0 cm3 of 0.5 mol dm-3 silver nitrate solution is reacted with 25 cm3 of 0.5 mol dm3 sodium chloride solution. As a result there is a change in temperature of the mixture and a white precipitate is formed. Before reaction After reaction Figure 4 3 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (b) Why is a polystyrene cup used in the experiment ? ………………………………………………………………………………………………………… [1 mark] (i) (ii) Based on the change of temperature in the experiment, state the type of reaction that occurred. ……………………………………………………………………………………………………. [1 mark] How is the total energy of the product different from the total energy of the reactants? ……………………………………………………………………………………………………. [1 mark] (c) State one step that should be taken while adding the two solutions in order to get a more accurate result. ………………………………………………………………………………………………………… [1 mark] (d) The ionic equation for the precipitation reaction of silver chloride is : Ag+(aq) + Cl-(aq) AgCl(s) (i) What is the number of moles of Ag+ ions that reacted with Cl- ions ? [1 mark] (ii) Calculate the heat change of the precipitation reaction that has taken place. Use the information that the specific heat capacity of water is 4.2 J g-1 C-1 and density water is 1 g cm-3. [2 marks] 4 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (iii) Calculate the heat of precipitation for this reaction. [2 marks] (e) The calculated value of the heat of precipitation for this reaction is less than the actual value. Give a reason. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… [1 mark] 3. SPM 2005/P2/Q3 A pupil carried out an experiment to determine the value of heat of displacement. Figure 3 shows the set up of the apparatus used in the experiment. Zinc Powder Glass cup Copper (II) sulphate Solution Figure 3 The following data was obtained : Initial temperature of copper(II) sulphate solution, 1 = 28 oC Highest temperature of the mixture of product, 2 = 48 oC (a) Complete the ionic equation for the reaction that occurred. Zn + Cu2+ …………………………………………………………………………………………. [1 mark] 5 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (b) In this experiment, excess zinc is added to 100 cm3 of 0.5 mol dm-3 copper(II) sulphate solution. Given that the specific heat capacity of the solution is 4.2 J g-1 oC-1 and the density of the solution is 1.0 g cm-3. (i) (ii) Calculate the change of heat capacity of the experiment. Use the formula, H = mc [2 marks] Calculate the heat of displacement in the experiment. The number of moles of copper(II) sulphate that reacted = ………………………………………………...... Heat of displacement (c) = …………………………………………………... [2 marks] Draw the energy level diagram for the reaction. [2 marks] (d) It was found that the heat of displacement value in (b)(ii) is not the same as the actual value. Suggest one step that must be taken to get a more accurate value. ………………………………………………………………………………………………………… [1 mark] (e) Based on the experiment, what is meant by the heat of displacement ? ………………………………………………………………………………………………………… [1 mark] (f) The pupil repeats the experiment, replacing the metal zinc with metal X. The following equation shows the reaction and the value of heat of displacement of metal iron and metal X. 6 Akhmalazmi86@blogspot.com Form 5 Chapter 3 Equation I : Fe (s) + CuSO4 (aq) FeSO4 (aq) + Cu(s), H = -150 kJ mol-1 Equation II : X(s) + CuSO4 (aq) XSO4 (aq) + Cu(s), H = - 100 kJ mol-1 Predict the metal X. Choose from this list : Aluminium, magnesium and tin. ………………………………………………………………………………………………………… [1 mark] 4. SPM 2005/P2/Q5 (a) What is the meaning of the heat of combustion of an alcohol? ………………………………………………………………………………………………………… [1 mark] (b) Table 5 shows the heat of combustion of three types of alcohol. The number of carbon atoms and the attractive force between molecules are among the factors that affect the value of heat of combustion. Name of alcohol Methanol Ethanol Propanol Molecular formula CH3OH C2H5OH C3H7OH Heat of combustion /kJ mol-1 725 1376 2015 Table 5 (i) Use data from Table 5 to draw the graph of the heat of combustion against number of carbon atoms on the graph paper provided. [2 marks] 7 Akhmalazmi86@blogspot.com (ii) Form 5 Chapter 3 Based on the graph in (b)(i), as the number of carbon atoms increases so does the value of the heat of combustion. Explain why. …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… [2 marks] 8 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (iii) Calculate the heat released when 2.3g of ethanol is completely burnt in air. Given that the relative atomic mass of C=12, H=1, O=16. Use the formula : Heat released = Number of moles X Heat of combustion. [2 marks] (c) Methanol and ethanol do not have isomers. Propanol has two isomers. Draw the structures of the two isomers of propanol. [2 marks] (d) Table 5.2 shows the freezing and the boiling points of mercury, methanol, ethanol and butanol. Substance Mercury Methanol Ethanol Butanol Freezing point /0C -39 -97 -117 -90 Boiling point /0C 357 64 79 117 Table 5.2 A thermometer may contain mercury or an alcohol. A mercury thermometer is not suitable to measure the temperature at around -100 0C. Name a suitable alcohol that can be used in a thermometer to measure the temperature at around –100 0C. Give one reason for your choice. Name of alcohol : ……………………………………………………………………….... Reason : ……………………………………………………………………….... ……………………………………………………………………….... ……………………………………………………………………….... [2 marks] 9 Akhmalazmi86@blogspot.com Form 5 Chapter 3 5. SPM 2006/P3/Q1 Diagram 1.1 shows two experiments to determine the heat of neutralisation. Experiment I Reaction between 25 cm3 of sodium hydroxide solution, NaOH, 2.0 mol dm-3 and 25 cm3 of ethanoic acid, CH3COOH, 2.0 mol dm-3. Initial temperature of mixture : ……………… °C Highest temperature of mixture : ……………… °C Change in temperature : ……………… °C Experiment II Reaction between 25 cm3 of sodium hydroxide solution, NaOH, 2.0 mol dm-3 and 25 cm3 of hydrochloric acid, HCl, 2.0 mol dm-3. Initial temperature of mixture : T1 °C Highest temperature of mixture : T2 °C Change in temperature : T3 °C Diagram 1.1 10 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (a) Write the initial and the highest temperature of the mixture and change in temperature for Experiment I in Diagram 1.1. [3 marks] (b) Construct a table that can be used to record the data from both experiments. [3 marks] (c) State one hypothesis for both experiments. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… [3 marks] (d) Based on the temperature in Experiment I, predict the predict the change in temperature in Experiment II. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… [3 marks] (e) Why must the initial temperature and the highest temperature be recorded in these experiments? ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… [3 marks] (f) How can the value of the changes in temperature be obtained? ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… [3 marks] (g) State three observations that you could obtain in Experiment I other than the change in temperature. 1………………………………………………………………………………………………………… 2………………………………………………………………………………………………………… 11 Akhmalazmi86@blogspot.com Form 5 Chapter 3 3………………………………………………………………………………………………………… [3 marks] (h) State three constant variables in this experiment. 1………………………………………………………………………………………………………… 2………………………………………………………………………………………………………… 3………………………………………………………………………………………………………… [3 marks] (i) Diagram 1.2 shows the calculation to determine the heat of neutralization for the reaction in Experiment I and Experiment II. Experiment I Experiment II Heat release = mc Heat release = mc = 50 g x 4.2 4.2 J g-1 oC-1 x ………… oC = 50 g x 4.2 4.2 J g-1 oC-1 x T3 oC = xJ = yJ Heat of neutralization Heat of neutralization = = x kJ _________________________________ Number of mole of water produced y kJ _________________________________ Number of mole of water produced Diagram 1.2 Based on Diagram 1.2 : (i) Give the operational definition for the heat of neutralization. ……………………………………………………………………………………………………. ……………………………………………………………………………………………………. [3 marks] (ii) It was found that the value of y is greater than the value of x. Explain why. ……………………………………………………………………………………………………. ……………………………………………………………………………………………………. [3 marks] (j) The experiment is repeated using the methanoic acid. The values of the heat of neutralization of these acids are given in Table 1. Complete Table 1 by classifying the acids as strong acid or weak acid. 12 Akhmalazmi86@blogspot.com Form 5 Chapter 3 Name of acid Ethanoic acid Heat of neutralization/kJ mol-1 -50.3 Hydrochloric acid -57.2 Mathanoic acid -50.5 Type of acid Table 1 [3 marks] 6. SPM 2007/P3/Q1 Diagram 1.1 shows the apparatus set-up for experiments I, II, III and IV. The magnification of the thermometers shows the readings of the initial temperature and the highest or lowest temperatures in each experiment. (a) (i) Record the temperature readings in the spaces provided in Diagram 1.1. [3 marks] Experiment I Experiment II Experiment III 13 Akhmalazmi86@blogspot.com Form 5 Chapter 3 Experiment IV Diagram 1.1 (ii) Construct a table to show all the data in each of these experiments. [3 marks] (iii) Classify the reactions in these experiments as either exothermic reactions or endothermic reactions. Exothermic reaction Endothermic reaction [3 marks] (b) A student repeated Experiment I several times. (i) State three things must be kept constant in these experiments. 1………………………………………………………………………………………………… 2………………………………………………………………………………………………… 3………………………………………………………………………………………………… [3 marks] 14 Akhmalazmi86@blogspot.com (ii) Form 5 Chapter 3 State the hypothesis for Experiment I. ……………………………………………………………………………………………………. ……………………………………………………………………………………………………. [3 marks] (c) Based on Experiment II : (i) Sate the temperature change and give two reasons for the change. Temperature change : ……………………………………………………………………………………………………. Reason1 : ……………………………………………………………………………………………………. Reason2 : ……………………………………………………………………………………………………. [3 marks] (ii) Sate the operational definition for the reaction that takes place. ……………………………………………………………………………………………………. ……………………………………………………………………………………………………. [3 marks] (d) The reaction in Experiment III is a neutralization reaction. Other acids can be substituted for hydrochloric acid. These acids have the same volume and concentration as the hydrochloric acid in Experiment III. Predict the temperature in the neutralization reactions of these acids. 1. Sulphuric acid : …………………………………………….. oC 2. Nitric acid : …………………………………………….. oC 3. Ethanoic acid : …………………………………………….. oC [3 marks] 15 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (e) Diagram 1.2 shows some observations in experiment IV. Diagram 1.2 (i) State three observations shown in Diagram 1.2. 1………………………………………………………………………………………………… 2………………………………………………………………………………………………… (ii) 3………………………………………………………………………………………………… [3 marks] The following chemical equation represents the reaction in Experiment IV. HCl(aq) + NaHCO3(aq) → NaCl(aq) + CO2(g) + H2O(l) Based on the chemical equation, and the answer in 1(e)(i), what inference can be made from Experiment IV? ……………………………………………………………………………………………………. ……………………………………………………………………………………………………. [3 marks] (iii) Sketch a graph to show the change in the volume of carbon dioxide gas produced against time. [3 marks] 16 Akhmalazmi86@blogspot.com Form 5 Chapter 3 7. SPM 2008/P2/Q6 The thermochemical equation for the neutralization reaction between nitric acid and sodium hydroxide solution is given below. HNO3 + NaOH NaNO3 + H2O , ∆H = -57.3 kJ mol-1 (a) State the meaning of heat of neutralisation. ………………………………………………………………………………………………………… [1 mark] (b) Based on the given thermochemical equation, state one observation when dilute nitric acid is added to sodium hydroxide solution. Explain your answer. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… (c) ………………………………………………………………………………………………………… [2 marks] In an experiment, 100 cm3 of 2 mol dm-3 nitric acid solution was added to 100 cm3 of 2 mol dm-3 sodium hydroxide solution. [Specific heat capacity of solution = 4.2 J g-1 oC-1; Density of solution = 1 g cm-3] Calculate (i) The heat energy released in this experiment, [2 marks] (ii) The temperature change in this experiment. [2 marks] (d) Draw the energy level diagram for the reaction between nitric acid and sodium hydroxide. [2 marks] 17 Akhmalazmi86@blogspot.com Form 5 Chapter 3 (e) Nitric acid and ethanoic acid both react with sodium hydroxide by a neutralisation reaction. HNO3 + NaOH NaNO3 + H2O , ∆H = -57.3 kJ mol-1 CH3COOH + NaOH CH3COONa + H2O , ∆H = -55.2 kJ mol-1 Explain why the heat of neutralisation for each reaction is slightly different. ……………………………………………………………………………………………………….. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… [2 marks] 18 Akhmalazmi86@blogspot.com Form 5 Chapter 3 CHAPTER 4 : THERMOCHEMISTRY 1. SPM 2003/P2/Q6 (a) Heat change when 1 mol of metal is displaced from its salt solution by a more electropositive metal. (b) Initial temperature and highest temperature. (c) 1. Stir the mixture. 2. Add the two solutions as quickly as possible. 3. Use polystyrene or plastic cup (any one) (d) (i) 1. Grey solid is deposited 2. Colourless solution turns blue 3. The thermometer reading rises or the container becomes hot or warm. (any one) (ii) (e) 1. Silver metal is produced 2. copper(II) ion is produced 3. exothermic reaction/ heat is released to the surroundings (i) = 0.5 x 100 1000 = 0.05 mol (ii) = 0.05 x 105 kJ = 5250 J (iii) Ө = 5.25 x 1000 100 x 4.2 = 12.5 oC (f) Energy + Cu + Ag -1 ∆H = -105 kJ mol 2+ Cu (g) + Ag 1. Mol of Ag+ = 1 x 100 = 0.1 mol 1000 19 Akhmalazmi86@blogspot.com Form 5 Chapter 3 = 25 oC 2. Heat change, Ө = 0.1 x 10500 100 x 4.2 3. Number of mol of Ag+ is double or concentration of silver nitrate is double. 2. SPM 2004/P2/Q4 (a) To reduce the heat loss to the surroundings. (b) (i) (ii) (c) Exothermic reaction Total energy of products is less than total energy of reactants Mix the solutions quickly and stir the reaction mixture. (d) (i) (ii) Number of moles Ag+ = 25 x 0.5 100 = 0.0125 mol The heat change = mcө = 50 x 4.2 x (31.5-29.0) = 525 J (iii) 0.0125 mol of Ag+ ions that reacted with Cl- ions released 525 J 1 mol of Ag+ ions that reacted with Cl- ions released = 525 J 0.0125 = 42000 J Heat of precipitation = -42 kJmol-1 (e) Heat is released to surroundings. 3. SPM 2005/P2/Q3 (a) Zn2+ + Cu (b) (i) (ii) ∆H = 100 x 4.2 x 20 = 8400 J Number of moles CuSO4 reacted = 0.5 x 100 1000 Heat of displacement = = 0.05 mol ___ mcө_______ Number of moles = 8400 0.05 = -168 000 J mol-1 = -168 kJ mol-1 20 Akhmalazmi86@blogspot.com (c) Form 5 Chapter 3 Energy 2+ Zn + Cu -1 ∆H = -168 kJ mol 2+ Zn + Cu 1. Use a plastic / polystyrene cup 2. add the zinc powder quickly. 3. stir the solution (d) (any one) (e) The heat released when 1 mole of copper is displaced from its solution. (f) Tin (Sn) 4. SPM 2005/P2/Q5 (a) The heat released when 1 mole of alcohol is completely burnt in excess oxygen. (b) (i) (ii) 1. 2. all points are transferred correctly draw a straight line The greater the number of carbon dioxide molecules, more products are formed which causes more heat to be released during the formation of bonds. (iii) Relative molecular mass of ethanol = (12 x 2) + (1 x 6) + 16 = 46 Number of moles ethanol = 2.3 = 0.05 mol 46 Heat released = 0.05 x 1376 = 68.8 kJ = 68 800 J (c) (d) - Ethanol - The freezing point of ethanol is -117 oC, which is lower than -100 oC. 5. SPM 2006/P3/Q1 (a) Initial temperature of mixture : 28.0 oC Highest temperature of mixture : 40.0 oC 21 Akhmalazmi86@blogspot.com Change in temperature Form 5 Chapter 3 : 12.0 oC (b) Experiment Initial temperature of mixture/ oC Highest l temperature of mixture/ oC Change in temperature/ oC (c) Experiment I 28.0 Experiment II T1 40.0 T2 12.0 T3 Strong acid produces higher heat of neutralization than weak acid. (d) 12.5 oC - 15.0 oC (e) To enable us to obtain the change in temperature for both experiments. (f) Change in temperature = Highest temperature of mixture - Initial temperature of mixture (g) 1. A colourless mixture of solution is obtained. 2. The vinegar smell of ethanoic acid disappears. 3. The polysterene cup becomes hot. 4. Thermometer reading is rises (h) 1. The volumes of the acid and the alkali. 2. The concentrations of the acid and the alkali. 3. The type of cup used in the experiment. (i) (i) The heat of neutralization is defined as the amount of heat released when 1 mole of water is produced. (ii) Experiment II uses a strong acid whereas Experiment I uses a weak acid. (j) Name of acid Ethanoic acid Hydrochloric acid Methanoic acid Type of acid Weak acid Strong acid Weak acid 6. SPM 2007/P3/Q1 (a) (i) Experiment I Experiment II Experiment III Experiment IV Initial temperature (oC) 28.0 29.0 27.0 30.0 Highest temperature (oC) 36.0 25.0 32.0 27.0 Initial temperature (oC) Highest temperature (oC) (ii) Experiment 22 Akhmalazmi86@blogspot.com Form 5 Chapter 3 I II III IV (iii) (b) (i) (c) 28.0 29.0 27.0 30.0 Exothermic reaction Experiment I Experiment III 36.0 25.0 32.0 27.0 Endothermic reaction Experiment II Experiment IV 1. The mass of sodium hydroxide. 2. the volume of water in the cup. 3. The size of the polystyrene cup. (ii) The reaction between sodium hydroxide and water is an exothermic reaction. (i) Temperature change = 4 oC Reason 1 : Heat energy is absorbed by the reactants from the surroundings. Reason 2 : The energy of the products is more than the energy of the reactants. (ii) The decrease in temperature shows that endothermic reaction happens where heat energy is absorbed from the surroundings. (d) 1. 37 oC 2. 32 oC 3. 30 oC (e) (i) (ii) 1. Final temperature is lower than the initial temperature. 2. The temperature reading decreases. 3. Bubbles of gas are released. Heat energy is absorbed when hydrochloric acid reacts with sodium hydrogen carbonate to produce sodium chloride, carbon dioxide and water. (iii) Volume of carbon dioxide gas,/cm3 Time /minute 23 Akhmalazmi86@blogspot.com Form 5 Chapter 3 7. SPM 2008/P2/Q6 (a) Heat change when 1 mole of hydrogen ions reacts with 1 mole of hydroxide ions to form 1 mole of water. (b) Observation : the mixture becomes hot or temperature increase Explanation : the reaction is exothermic (c) (i) No. of moles of NaOH = 100 x 2 = 0.2 mol 1000 Energy released = 0.2 x 57.3 = 11.46 kJ (ii) (d) Temperature change = 11.46 x 1000 200 x 4.2 = 13.6 oC Energy NaOH + HNO3 -1 ∆H = -57.3 kJ mol Na NO3 + H2O (e) 1. Ethanoic acid is a weak acid which partially ionize in water, nitric acid is strong acid that ionize completely in water. 2. energy is used to ionize/dissociate weak acid. 24









