The Aldol Condensation: Synthesis of a Chalcone
advertisement

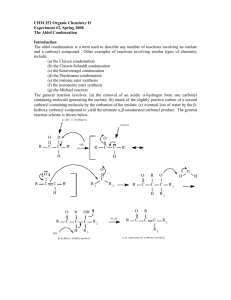
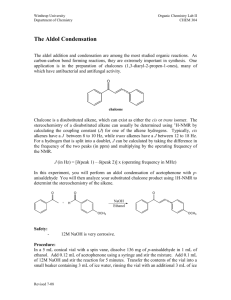
CH421 Experiment 6 The Aldol Condensation: Synthesis of a Chalcone Reading: Organic Chemistry by John McMurry, 8e, Chapter Sections 11.10 (E1cB mechanism) and 23.1‐23.5 (Aldol condensation) Techniques: Recrystallization, TLC, IR, NMR Introduction Aldol condensations represent an important class of carbon‐carbon bond formation reactions both in nature and in synthetic chemistry. Compounds called chalcones (or chalconoids) can be prepared by the aldol condensation of an aromatic ketone and an aldehyde. In a chalcone, two aromatic rings are joined by a 3‐carbon ,‐unsaturated carbonyl system, as illustrated below. Recall that the relative positions of carbons (and their corresponding hydrogens) in a carbonyl compound are designated using Greek letters. The positions are labeled as , , , , and , etc. as they get further away from the carbonyl carbon. Chalcones are a subset of compounds known as flavonoids. Flavonoids are produced naturally in plants and are often responsible for the color of leaves and flowers. Many naturally‐occurring flavonoids have been shown to exhibit anti‐ oxidant and anti‐inflammatory properties, and thus synthetic chalcones have potential applications in medicine, as well as other possible uses. Originally, it was discovered that when acetaldehyde is treated with base at room temperature or below, it undergoes a self‐condensation reaction to form 3‐hydroxybutanal. When this reaction was first studied in the early nineteenth century, the product, 3‐hydroxybutanal, was named “aldol” as a reflection of the aldehyde‐alcohol components of its structure. In the presence of heat and base, the 3‐hydroxybutanal undergoes further elimination of water to form the ,‐unsaturated aldehyde, trans‐2‐butenal. Today, the definition of an aldol reaction has been expanded to include the reaction of any enolate (regardless of origin, i.e., aldehyde, ketone, amide, ester, etc.) and an aldehyde. Both aldehydes and ketones that bear a hydrogen on the ‐ carbon readily form enolate ions in the presence of base because the electron‐withdrawing effects of carbonyls render the ‐hydrogens slightly acidic. The aldol condensation of acetaldehyde shown above illustrates a “self‐condensation.” An aldol condensation between two different aldehydes or ketones is called a crossed aldol condensation. Such condensations can result in an undesirable mixture of products, since both reactants can undergo self‐condensation as well as crossed condensation. However, in some cases, crossed aldol condensations do yield a single product in good yield, as in the synthesis of chalcones. Experiment 6: The Aldol Condensation: Synthesis of a Chalcone In this experiment, you will perform a crossed aldol condensation reaction with an unknown para‐substituted acetophenone and an unknown para‐substituted benzaldehyde under basic conditions to provide a chalcone product (see Table 1 below for structures of unknowns). This particular crossed aldol reaction is expected to proceed cleanly since only the substituted acetophenone can form an enolate, as the substituted benzaldehyde does not contain any ‐hydrogens. Under these equilibrating reaction conditions, the acetophenone enolate is formed in low concentration, since the equilibrium favors the reactants (this would be expected based upon the reactant and product pKa values given – see reaction scheme below). The nucleophilic acetophenone enolate then reacts with the substituted benzaldehyde, rather than another acetophenone molecule, since aldehydes are more electrophilic than ketones and thus react much more rapidly. As is true of most aldol condensations (especially those involving aromatic aldehydes), the aldol addition product readily undergoes dehydration (via E1cB elimination) to give an ,‐unsaturated carbonyl compound. The double bond formed is then part of a conjugated system with both the carbonyl group and the aromatic rings. Since this reaction is performed under equilibrating conditions, the lower energy alkene product is obtained – specifically, the highly conjugated trans‐alkene. The formation of the highly conjugated alkene product thus serves to drive the equilibrium to the product side. We will use two solvents in today’s experiment. The substituted benzaldehyde and acetophenone are soluble in EtOH, whereas NaOH is soluble in water. Hence, a co‐solvent system is used in this reaction. The aldol condensation product is less soluble than the starting materials, so it precipitates from the reaction mixture as it is formed. After the reaction is complete, addition of water promotes additional precipitation of the product (the organic compound is even less soluble in water than EtOH). The chalcone is thus isolated via filtration and then purified by crystallization from EtOH. Table 1: Possible structures of the unknown substituted acetophenones and substituted benzaldehyes. Substituted Acetophenone Name Substituted Benzaldehyde Name acetophenone p‐methoxybenzaldehyde (p‐anisaldehyde) 4’‐methylacetophenone p‐methylbenzaldehyde (p‐tolualdehyde) Safety Precautions Ethanol is flammable. NaOH is caustic – if any is accidentally spilled on your skin, wash with excess water. Experiment 6: The Aldol Condensation: Synthesis of a Chalcone Pre‐Lab Notebook (to be completed prior to the start of lab) Enter date, experiment number and title on notebook page and in TOC with corresponding page number. Copy the reaction and the reaction table below (in your own writing) into your notebook and complete all empty cells in the table. Perform the mmol calculations first for all reactants, including both possible substituted acetophenones and both substituted benzaldehydes. Then calculate the equivalents for the aldehydes using acetophenone as the limiting reagent (1.0 equiv). NOTE: fill in the product row only after you have determined the structure of your product in order to calculate theoretical yield. Make sure to check your calculations with your instructor before starting the experiment. Name MW (g/mol) mass (g) mmol equiv d (g/mL) vol (mL) mp (°C) bp (°C) acetophenone (R = H) 120.15 ____ 1.0 1.03 0.60 ____ ____ 4’‐methyl‐ acetophenone (R = CH3) 134.18 ____ 1.0 1.00 0.60 ____ p‐tolualdehyde (R’ = CH3) 120.15 ____ 1.02 0.65 ____ p‐anisaldehyde (R’ = OCH3) 136.15 ____ 1.12 0.65 ____ ____ 50% NaOH w/v 40.00 ____ ____ 0.50 ____ ____ chalcone product Theor. mass = Theor. mmol = ____ _____ ___ ____ ___ Experiment 6: The Aldol Condensation: Synthesis of a Chalcone Procedure Aldol Condensation To a 50 mL Erlenmeyer flask with a stir bar, add 0.65 mL of unknown substituted benzaldehyde and 0.60 mL of unknown substituted acetophenone. Make sure to record the unknown letter and number that you used for the acetophenone and aldehyde unknowns. Dissolve these starting materials in 4 mL of 95% EtOH. Next, add 0.6 mL of a 50% NaOH solution to the flask, and allow the reaction mixture to stir for 10 minutes. During this time, you should observe the formation of a solid, which is the chalcone product. If a solid is not observed after 10‐15 minutes, gently scratch the inside of the flask with a metal spatula. After the reaction mixture has stirred for 10‐15 minutes, and a solid is present, add 15 mL of ice water to the flask. Gently break up the solid with a spatula, then filter using a Buchner funnel. Wash the product with cold water (3 x 5 mL) and then dry the product. Put a few specks of the crude solid from the funnel into a clean sample vial labeled “crude” and dissolve in 1 mL of EtOAc for a TLC to be run later (see below). Product Purification Recrystallize the crude product from hot 95% EtOH (bp 78 °C). An estimated 4 mL of EtOH per gram of solid is required. Place a few specks of your purified solid in a clean sample vial labeled “pure” and dissolve in 1 mL EtOAc for TLC analysis. TLC Analysis Mark a TLC plate with 3 tick marks: “crd” on the left, co‐spot in the center, and “pure” on the right, and spot with the corresponding TLC sample solutions prepared above. Develop the TLC plate in 4:1 hexanes:ethyl acetate. Draw this plate and calculate Rf values for all spots in your Data section. Waste Aqueous Waste #1: the aqueous filtrate from the initial isolation of the crude chalcone. Organic Waste #2: recrystallization filtration, NMR solution, and all acetone rinses (glassware, sidearm flask with crude product, NMR tube, etc.) Solid Chemical Waste #4: filter paper Data (collect and record in your notebook during the lab) Describe product, give mass of product isolated, and calculate % yield (show calculation). TLC data (draw TLC, calculate Rf’s of all spots, and include mobile phase) Obtain IR (solid is best), staple in notebook, and assign major functional group peaks. Obtain 1H NMR spectrum (CDCl3), label as “Name and Name Expt 6 purified prod in CDCl3,” and staple into notebook. Draw the structure of the product on the NMR spectrum, and label all peaks. Conclusion (record in your notebook) State outcome, yield, specific proof of product structure (and thus structures of the unknown acetophenone and benzaldehyde starting materials) based on both IR and NMR, comment on purity (based on TLC and NMR), possible sources of error, and any other information deemed relevant. The IR and 1H NMR spectra for all starting materials are given below for your reference. Experiment 6: The Aldol Condensation: Synthesis of a Chalcone IR of acetophenone (CCl4 solution) S, 3H 1 H NMR acetophenone (90 MHz, CDCl3) d, 2H m, 3H Experiment 6: The Aldol Condensation: Synthesis of a Chalcone IR of 4’‐methylacetophenone (thin film) S, 3H S, 3H d, 2H 1 H NMR 4’‐methylacetophenone (400 MHz, CDCl3) S, 3H 1 H NMR p‐tolualdehyde (90 MHz, CDCl3) s, 1H d, 2H d, 2H Experiment 6: The Aldol Condensation: Synthesis of a Chalcone S, 3H 1 H NMR p‐anisaldehyde (90 MHz, CDCl3) s, 1H d, 2H d, 2H