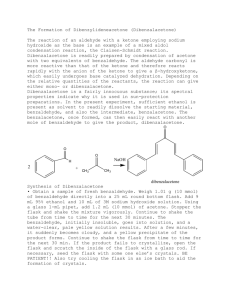
GENERAL PROCEDURE FOR THE SYNTHESIS OF CHALCONESa
advertisement

GENERAL PROCEDURE FOR THE SYNTHESIS OF CHALCONESa Reference: Roberts, Gilbert, & Martin, "Experimental Organic Chemistry," Saunders, 1994, 543. Please calculate the amounts you need prior to coming to the lab so you can spend your lab time doing chemistry rather than calculations! O O O H CH 3 NaOH/EtOH/H2O + X Y Y benzaldehyde acetophenone X chalcone Dissolve 0.01 mol of the benzaldehyde and 0.01 mol of the acetophenone in 10 mL of 95% ethanol in a 25-mL Erlenmeyer flask equipped with a magnetic stirring bar b. 3.5 mL of 6M NaOH solution are then added to the reaction flask using a Pasteur pipettte. Stir the reaction mixture for 10 minutesc. Cool in an ice-water bath until crystal formation is complete. Add 2 mL of ice-cold water to the flask and vacuum filter. Wash the crystals with 5 mL of water followed by 3-5 mL of ice-cold ethanol. Allow to air-dry. Recrystallize from 95% ethanol if necessaryd. Notes a Not all combinations of substituted benzaldehydes and acetophenones will result in the simple formation of chalcones. Try figuring out which combinations should be more reactive. Some will require longer reaction times (e.g., the unsubstituted chalcone takes approximately 3 hours and usually forms an oil rather than crystals. However, a recent JCE article (2000, Vol. 77, p. 511) gives a method that apparently works. By all means give this variation a try. You can get the article from the lab web site). Use TLC to determine the appropriate reaction time. Some combinations will not work. b Some benzaldehydes and acetophenones are not very soluble in ethanol. If this is the case you can a) use some more ethanol and/or b) warm up the solution. Since the Aldol condensation is exothermic be careful if adding the NaOH solution to a warm solution of the carbonyl compounds. c Often the chalcone will begin to precipitate before the 10 minutes is up. However, don't cool until the ten minutes is up. If the chalcone has not begun to precipitate after 10 minutes, check the progress of the reaction by TLC before cooling. Do not throw away the reaction mixture as "no good." See note a above. d Check the purity of your product by TLC (7:3 hexane/ethyl acetate on silica gel is a good place to start. Remember to run starting materials on the same plate as your sample.)