Introduction to Atmospheric Sciences
advertisement

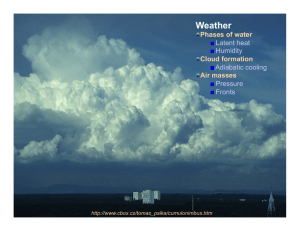
Unit 6 Phase transitions Nicole Mölders Heterogeneous system Tv ρv pv Tv ρv pv gaseous Tv ρv pv gaseous Ts ρs ps Tl ρl pl liquid Homogeneous Heterogeneous solid Tl ρl pl liquid Heterogeneous Phase transitions equilibrium Thermodynamic properties of water ⇒ µ= µ (p,T)= µ‘ (p’,T’) ⇒ p=f(T) or T=f(p) ⇒ In one-component system with 2 phases, 1 independent variable exists! Latent heat dU=L-pdV=L-RvT dV=Vv-Vwi~Vv for vaporization & sublimation dV~0 for fusion Clausius-Clapeyron-equation Joule’s law TdS=dU+pdV Analystical solution of the ClausiusClapeyron-equation lv=const. vw<<vv → ∆v~vv With lv=2.5* 106J/kg, Rv=461.5 J/(kg K), esw,o=6.1078hPa, To=273.15K ⇒lv/(RvTo)=19.81 Stüve diagram isotherms isobars http://en.wikipedia.org/wiki/File:Stuve-diagram.gif Relative humidity is a relative measure for the degree of saturation Hair hygrometer measures relative humidity Sling psychrometer for measuring humidity by use of Temperature and dewpoint temperature http://www.infoplease.com/images/cig/weather/21fig05.png http://en.wikipedia.org/wiki/Hygrometer#mediaviewer/File:HaarHygrometer.jpg Dew-point temperature gradient http://ww2010.atmos.uiuc.edu/guides/mtr/svr/modl/line/gifs/squall3.gif http://ww2010.atmos.uiuc.edu/guides/mtr/af/frnts/gifs/home.gif Vertical mixing requires to know your extensive and intensive quantities for correct calculations P1 ∆p P p2 h http://www.susanstevenson.com/Journal/2013/November/90210PushingCarOffCurbP.jpg