Chapter 17 Lecture Notes
advertisement
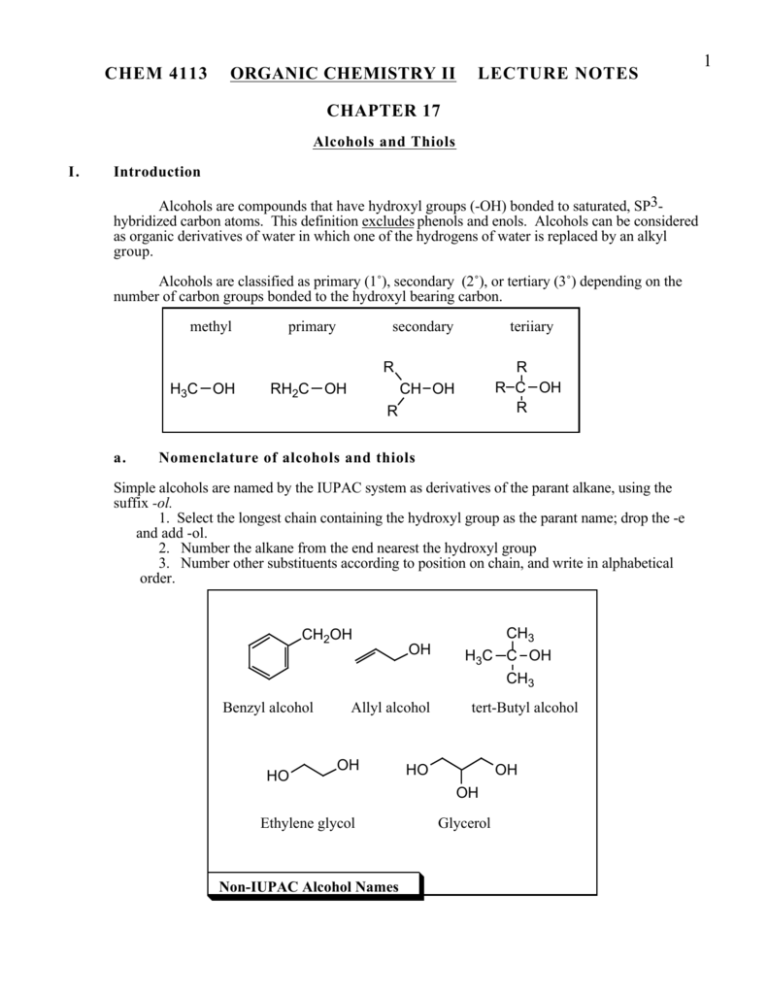
CHEM 4113 ORGANIC CHEMISTRY II LECTURE NOTES CHAPTER 17 Alcohols and Thiols I. Introduction Alcohols are compounds that have hydroxyl groups (-OH) bonded to saturated, SP3hybridized carbon atoms. This definition excludes phenols and enols. Alcohols can be considered as organic derivatives of water in which one of the hydrogens of water is replaced by an alkyl group. Alcohols are classified as primary (1˚), secondary (2˚), or tertiary (3˚) depending on the number of carbon groups bonded to the hydroxyl bearing carbon. methyl primary secondary teriiary R H3C OH RH2C OH R R C OH R CH OH R a. Nomenclature of alcohols and thiols Simple alcohols are named by the IUPAC system as derivatives of the parant alkane, using the suffix -ol. 1. Select the longest chain containing the hydroxyl group as the parant name; drop the -e and add -ol. 2. Number the alkane from the end nearest the hydroxyl group 3. Number other substituents according to position on chain, and write in alphabetical order. CH2OH Benzyl alcohol HO OH Allyl alcohol OH CH3 H3C C OH CH3 tert-Butyl alcohol HO OH OH Ethylene glycol Non-IUPAC Alcohol Names Glycerol 1 2 Alcohols are named from the parent alkane: 1. drop the -e and add -ol 2. hydroxyl has priority and gets lowest possible no. OH OH 2,6-octanediol (not 3,7-octanediol) NOTE: polyalcohols retain the -e ending. OH OH trans-4-hexen-3-ol NOTE: OH higher priority than olefin. CH3 trans-2-methylcyclohex-4-en-1-ol Thiols are named from the parent alkane with the suffix thiol SH SH OH 5-methyl-2-heptanethiol 7-mercapto-4-decanol NOTE: OH higher priority than SH; mercapto is the designator of SH substituent. Nomenclature of alcohols and thiols. b. Properties of alcohols Alcohols are different from hydrocarbons and alkyl halides. One example of quite different physical properties is boiling point. Alcohols have much higher boilong points than other compounds of similar molecular weight. 3 OH H3C H 3C ethanol CH3 propane bp degrees C 78 O H 3C H 3C CH3 dimethyl ether -48 ethyl flouoride -23 Hydrogen Bond: An interaction of an unshared pair on one molecule with the polarized OH bond of another. F -38 −δ +δ O H H O +δ −δ The Boiling Point of Alcohols is due to Hydrogen Bonding The reason for the higher boiling points is that alcohols, like water, are highly associated in solution because of Hydrogen bonding. Although H-bonds have typical strengths of 5 Kcal/mole, this means that extra energy must be added to break them during the boiling process. Alcohols as show significant solubility in water solutions. Alcohol, like water is a "Protic" solvent (that is, it can donate a hydrogen bond). "Like dissolves like", means that alcohols and water are miscible. However, once a certain number of carbons in the alkyl chain of the alcohol is reached, the alcohol is no longer soluble. This is the Six Carbon Rule. CH3(CH2)nCH2-OH n= Water solubility 0 1 2 3 4 5 miscible miscible 8 wt % 4 wt % 0.5 wt % insoluble Water Solubility as a Function of Chain Length. c. Acidity of alcohols and thiols The O-H and S-H bonds of alcohols and thiols are able to undergo homolytic cleavage to give a proton and the conjugate base. Thus, these functional groups are organic acids, although relatively weak organic acids. Cleavage of the O-H bond of an alcohol (R-OH) gives an alkoxide anion (R-O-), whereas cleavage of the S-H bond of a thiol (R-SH) gives a mercaptide anion (R-S- ) The thiols are somewhat stronger acids than the alcohols (by 5-6 pKa units) due to the weaker strength of the S-H bond. 4 H+ R O H + R O alcohol pKa (CH3)3COH CH3CH2OH HOH CH3OH CF3CH2OH (CF3)3COH WEAKER ACID 18.00 16.00 15.74 15.54 12.43 5.4 STRONGER ACID Acidity Constants of Some Acids The acidity of alcohols and thiols can be increased by the presence of neighboring electron withdrawing groups such as halogens. The electronegative group causes a dipole which inductively withdraws electron density from the OH bond, thus weakening it. This "Inductive Effect" falls of with increasing separation of the OH and halogen. F CH2 CH2 base OH F CH2 CH2 O Electronegative groups will withdraw electrons (polarize the bonds) along the sigma system. This will weaken the OH bond of the alcohol which increases its acidity. This effect also stabilizes the conjugate base (alkoxide anion). This effect decreases rapidly as the electronegative group is moved further away along the hydrocarbon chain. F3C (CH2)n OH n= 1 2 3 4 pKa 12.4 14.6 15.4 16.0 ( same for n-pentanpl) Effect of Electron-Withdrawing Groups on Alcohol Acidity Because of the relatively weak acidic nature of alcohols, very strong bases much be used to quantitatively convert (i.e.,>99%) the alcohol into the alkoxide anion. Typical bases such as Sodium hudroxide (NaOH) give only about 50% conversion. Strong bases such as Sodium Hydride (NaH) and Sodium Amide (NaNH2) are most often used. 5 C2H5OH + NaOH pKa = 16 C2H5ONa + Keq = 1 C2H5OH + NaH HOH pKa = 16 C2H5ONa + H2 pKa = 16 Keq = 1026 pKa = 42 Sodium Hydride (NaH) results in complete deprotonation Deprotonation of Alcohols Due to their greater acidity, thiols are converted into the mercaptide anion quantitatively through the use of Sodium Hydroxide (NaOH). C2H5SH + NaOH pKa = 10 C2H5SNa Keq = 106 + HOH pKa = 16 Thiols are stronger acids than alcohols and are completely deprotonated by Sodium Hydroxide (NaOH). Deprotonation of Thiols II. Synthesis of Alcohols Alcohols occupy a central position in organic chemistry, and can be synthesized from a variety of functional groups (alkenes, alkyl halides, aldehydes, ketones and esters, among others). Many of the routes to primary and secondary alcohols are summarized below. O B CH 3CH 2MgBr + HCH O A CH 3CH 2=CH 2 Hg(OAc)2 THF H2O CH 3CH 2C-OH H2O BH3 H3O+ I H3O+ O NaBH4 CH3 CH2 C-H O OH + H3O CH 3MgBr II H3O+ NaBH 4 ethanol O LiAlH4 CH CH C-OCH3 D 3 2 ether + CH 3CH 2CH 2OH H3O CH 3CHCH3 C LiAlH4 ether OH— H2O2 (1) NaBH 4 (2) H3O+ . + E HCCH3 C O CH 3CCH3 B Let's review briefly some of the methods of alcohol preparation we have already learned in Organic I lecture\ SN2 Substitution of primary and secondary alkyl halides Cl Cl + NaOH acetone OH + NaCl + CH3COO- OOCCH3 NaOH, H2O OH SN1 Substitution (Solvolysis) of tertiary alkyl halides Cl H2O NaHCO3 Synthesis of Alcohols from Alkyl Halides OH + NaCl 6 7 Electrophilic addition to alkenes BH3 CH2Cl2 Hg(OAc)2 H2O HOH2O OH primary alcohol NaBH4 secondary alcohol OH Synthesis of Alcohols from Alkenes Oxidation of alkenes H OH KMnO4 NaHSO3 OsO4 - OH H2O OH H cis diol Epoxidation of alkene and ring-opening H H H2O2 O OH H3O+ H H OH trans diol Preparation of Diols from Alkenes a. Alcohols via reduction of carbonyl compounds Organic "reduction" reactions are considered to be reactions which either increase the hydrogen content of a compound or reduce the oxygen, nitrogen or halogen content of a compound. Aldehydes and ketones are easily reduced to yield alcohols. Aldehydes produce primary alcohols and ketones give secondary alcohols. Polyhydride metal salts such as Sodium borohydride (NaBH4) and Lithium aluminum hydride (LiAlH4) are very effective reducing agents for this process. For aldehydes and ketones, NaBH4 is usually the reagent of choice because of the ease of use (LiAlH4 is much more difficult to work with). These reagents transfer a "hydride" to the carbonyl carbon, the resulting alkoxide anion is protonated with dilute aqueous acid. Since both reagents contain four hydrides, the intermediates produced from the initial reaction can undergo subsequent addition until all four hydrides have been used. 8 Metal Hydride Reducing Agents H H Al H H Li Na Lithium Aluminum Hydride LiAlH4, LAH R R O C O C Sodium Borohydride NaBH4 OH C H R Z OH C H R Z 1) LiAlH4, ether 2) H2O Z H H B H H NaBH4, MeOH Z Z = R or H Primary Alcohols Secondary Alcohols Aldehydes Ketones Hydride Reduction of Aldehydes and Ketones Lithium ion from LAH serves as Lewis Acid to activate carbonyl toward addition Li H O R C Al Z 1.0 equiv. H O Li C H R Z H ether H 0.25 equiv. (4 available hydrides) H Reaction product is H Al still an active reducing agent ( 3 more available H hydrides). H2O OH A total of 4 carbonyl compounds C H are reduced for each LAH R Z Reduction Mechanism Using LAH Hydrogen bond activates the carbonyl toward hydride addition H-OMe H H Reaction product is H H OH O still an active reducing B B MeOH agent ( 3 more available C H H hydrides). C R Z H H R Z 1.0 equiv. 0.25 equiv. (4 available hydrides) NaB(OMe)4 + Sodiumborohydride Reduction OH A total of 4 carbonyl compounds C H are reduced R Z 9 Like aldehydes and ketones, esters can be reduced to an alcohol through the use of metal hydride reagents. However, this process is more difficult and requires LAH which is a more reactive reagent. The process converts an ester into a primary (1˚) alcohol. The mechanism has been shown to occur in two discrete hydride addition steps. The first hydride addition leads to an aldenyde intermediate, which is immediatly reduced further to the alcohol, the aldehyde never builds up in solution. R R O C O C OR' 1) LAH, ether 2) H2O RCH2OH Reductions of esters with LAH results in formation of 1˚ alcohols. NaBH4, MeOH NO REACTION! OR' Mechanism of LAH reduction of esters Li R O C OR' R O C OR' R O C OR' O LAH, ether SLOW Ester -Donation of the lone-pair electrons from the methoxy group decreases the positive charge on the carbonyl carbon. This makes esters less reactive toward hydride addition than are ketones and aldehydes. C R H OR' Intermediate is unstable with respect to loss of MeOLi. - MeOLi Li OH C H R H H2O O C H R H LAH, ether FAST R O C H Differential Reactivity of Esters with Hydride Reagents b. Alcohols via Grignard Addition to Carbonyl Compounds. The addition of a Grignard reagent to a carbonyl compound, followed by treatment with a dilute acid yields an alcohol. Addition of a Grignard (RMgX) to formaldehyde (HC=OH) gives a primary alcohol RCH2 OH, addition to an aldehyde (R'C=OH) gives a secondary alcohol RR'CHOH, and addition to an ester (R'C=OOR") or ketone (R'C=OR') gives a tertiary alcohol RR'R'COH. Carboxylic acids do not give Grignard addition products. The Grignard reaction is sometimes limited by the fact that Grignard reagents can not be formed from starting materials that contain a reactive functional group such as a hydroxyl group. This problem can sometimes be corrected by protecting the functional group. Alcohols can be protected by the formation of trimethylsilyl (TMS) ethers, which are inert to Grignards and can be easily converted back to the alcohol. 10 O C Y O- O C Z Y The electrophilic carbon of the carbonyl group is susceptible to attack by nucleophiles such as hydrides and Grignards C Y + Z Z Resonance Hybrid Carbonyl Reactivity Synthesis of 2˚ and 3˚ alcohols OH R' Aldehydes C R Z Ketones O 1) R'MgX, ether C + R Z 2) H3O Z = R or H 2˚ alcohols 3˚ alcohols Mechanism + R O C MgX MgX R' Z - ether R O C R' Z O MgX H3O+ R' C R Z OH R' C R Z Esters and Carboxylic Acids R R O C O C 1) 2 equiv.R'"MgX, ether + OR' 2) H3O 1) 2 equiv.R'"MgX, ether + OH 2) H3O OH R'' Ester C R R'' 3˚ alcohol NO REACTION: Grignard reagents react with the proton of carboxylic acids. Grignard Reagent Addition to Aldehydes, Ketones and Esters 11 OH OH Proposed O H Synthesis Target Molecule PROBLEM: OH + MgBr The Grignard will react with the weakly acidic alcohol hydrogen in the substrate. This will quench the Grignard reagent, bringing the reaction to a halt. SOLUTION: "Protect" the alcohol as the trimethylsilyl ether. The ether is unreactive towards the Grignard Reagent, and the alcohol can be easily regenerated. OH Cl H3C Si CH3 CH3 O H OH R3N OH Si(CH3)3 O H 1) CH3CH2MgBr ether 2) H3O+ "Protected" alcohol H3O+ Si(CH3)3 OH Protection of Alcohols 3. Reactions of Alcohols Reactions of alcohols can be divided into two groups- those that occur at the C-O bond and those that occur at the H-O bond. Below is a summary of the various reactions that alcohols undergo. 12 O CH 3CH 2CH 2O S CaBr 2 S N2 CH 3 CH 3CH 2CH 2Br O tosyl chloride pyridine CH 3CH 2=CH 2 O PBr3 S N2 CH 3CH 2C-OH CrO3 POCl3 H2SO 4 H2O pyridine O CH 3CH 2CH 2OH POCl3 pyridine PCC CH 2Cl 2 CH 3CH 2C-H SOCl2 S N2 O CH 3CCH3 O Na CrO3 H2SO 4 H2O OH Cl CH 3CHCH3 NaH CH 3CHCH3 a. CH 3CH 2CH 2Cl SOCl2 S N2 PBr3 S N2 CH 3CHCH3 Br CH 3CHCH3 Dehydration of Alcohols to Alkenes One of the most important C-O reactions is dehydration to the alkene. In this process, the C-O bond is broken and a -bond is formed. One of the most common methods of dehydration is acid catalyzed dehydration. In this process, a strong acid such as H2 SO4 protonates the hydroxyl group, thus converting it into a good leaving group (-OH2 +). Loss of water by breaking the C-O bond generates a carbocation, with subsequent loss of an adjacent proton and formation of the -bond. The reaction occurs by an E1 mechanism. This process works extremely well with 3˚ alcohols, which will readily dehydrate and room temperature or even lower. However, 2˚ alcohols require more forceful condition, such as temperatures of 100˚ C. This is because the less stable 2˚ carbocation intermediate is slower to form. Primary alcohols are even less reactive and require very harsh conditions. As a result, this is not the preferred reaction for 1˚ alcohols; the best method is dehydration with POCl3 in pyridine solvent. Acid catalyzed dehydrations follow Zaitsev's rule, that is they will give the most stable alkene (the most substituted alkene) as the major product. If the intermediate carbocation of a 2˚ alcohol can rearrange to a more stable 3˚ carbocation, it will do so, and the major products will derive from this intermediate. 13 OH β1 OH2 H+ β2 -H2O Loss of water generates a carbocation intermediate Protonation of OH generates good leaving group (H2O) β3 E1 loss of a β proton to generate the olefin... three different sites of elimination are possible -H+ from β1 -H+ from β2 -H+ from β3 Note that this reaction is the reverse of alkene hydration reaction; that is, we could start with the alkene and run the reaction in the other direction to produce the alcohol. Whether we end up with the alkene or alcohol depends upon the reaction conditions. For example, we could shift the equilibrium by removing the lower boiling alkene by distillation. Dehydration of Alcohols to Alkenes via AcidCatalysis OH H3C C H3C H3C C H H+ cat. CH3 H3C C H3C H3C C H CH3 β H loss of H from β H3C C H3C H3C + C 3% CH2 2˚ carbonium ion CH3 The methyl moves with its pair of electrons. CH3 H3C C H3C H3C C H CH3 C H3C β2 H3C β2 C H β1 CH3 3˚ carbonium ion more stable H3C loss of H+ from β1 H3C H3C loss of H+ from β2 C CH2 Rearrangement of 2˚ Carbocations Prior to Alkene Formation OH O Cl P Cl Cl OPOCl2 Pyridine H Dehydration With POCl3 N C E2 Mechanism 61 % C CH3 CH3 C H CH3 36 % 14 Ease of Dehydration R H R R C OH > R C OH > R C OH R H H Temperatures necessary for reaction room temp and below 100-150˚C above 150˚C Because the acid catalyzed dehydration of an alcohol to form an alkene occurs via a carbocation, 3˚ and 2˚ alcohols react much more readily and under milder conditions than 1˚ alcohols. Rate of Acid Catalyzed Dehydration of Alcohols b Conversion into Alkyl Halides A second C-O bond reaction that alcohols undergo is conversion into alkyl halides when treated with hydrohalic acids (HCl or HBr). The first step in this reaction is protonation of the hydroxyl group, converting it into a good leaving group (H2 O). Tertiary alcohols then ionize to the 3˚ carbocation which undergoes an SN1 reaction with X -. Primary alcohols react by an SN2 displacement of water from the substrate by Cl-. Secondary alcohols mya react by either an Sn1 or SN2 mechanism depending on the structure of the 2˚ akcohol. 15 When both Z and A' are Z = R or H OH H alkyl groups, the tertiary OH carbocation is formed HCl Z Z' Cl Z Cl For secondary alcohols (one Z = H) either SN1 or SN2 pathways may operate For Z's both H; this protonated alcohol intermediate undergoes backside addition of Cl via the SN2 pathway Z' - H2O Z 'Z' Cl E1 Cl HH Cl Excess HCl SN1 Cl R RH RR R SECONDARY PRIMARY TERTIARY Any alkene formed by an E1 process will eventually be consumed by excess HCl. The equilibrium will be drained to the 3˚ chloride. Conversion of Alcohols to Halides Using HX Tertiary alcohols are readily converted even at temperatures as low as 0˚C. Primary and secondary alcohols react with much more difficulty, and are best converted into halides by treatment with SOCl2 and PBr 3 . The reactions of 1˚ and 2˚ alcohols with SOCL2 and PBr3 occur by an SN2 process. Hydroxide is too poor a leaving group to be displaced directly by a halide anion in an SN2 reaction. The above reagents convert the alcohol into a much better leaving group, that is easily expelled by a backside nucleophilic attack. Cl Thionyl chloride S O OH Cl N Chlorosulfite ester O O Cl S Cl + Cl O O S + N H Cl Need one chloride anion to act as nucleophile; this species keeps being regenerated as the chlorosulfite ester decomposes to sulfur dioxide and another chloride anion. Conversion of Alcohols to Alkyl Chlorides with Thionyl Chloride c. Conversion of Alcohol functional Group into Sulfonate Esters Alcohols are not good leaving groups in organic synthesis. In order to convert the alcohol OH into a better leaving group we often protonate it with a strong acid. We 16 cannot always use strongly acidic conditions to carrry out conversions of the alcohol functional group. Often times we can employ cleavage reactions of the alcohol O-H to convert the hydroxyl group into a much better leaving group as was done when POCL3 , SOCL2 and PBR3 are employed, but these are not general reactions. One particularly useful conversion is to transform the alcohol into a sulfonate ester by treatment of the alcohol with a sulfonyl chloride. Sulfonate derivatives have about the same leaving group ability as do halides. The p-toluenesulfonate esters derived from alcohols (tosylates) serve nicely as substrates in both elimination and substitution reactions. O Cl S R O R'OH/base O R' O S R O good leaving group sulfonate ester Note: abbreviation of tosylate ester is ROTs sulfonyl chloride O Cl S O CH3 R'OH/base O R' O S O CH3 p-toluenesulfonate ester (tosylate ester) p-toluenesulfonyl chloride (tosyl chloride) Inversion of chirality at chiral alcohol H OH (R)-3-heptanol TsCl pyridine NaOH H OTs tosylate ester acetone SN2 + OTs HO H (S)-3-heptanol Formation and use of Tosylate Esters d. Oxidation of Alcohols to Carbonyl Compounds Using Chromium (+6) Reqagents The most important reaction of alcohols is their oxidation to carbonyl compounds by Cr (+6) oxidizing agents such as Jones' Reagent (CrO3 /H2 SO4 ), Na2 Cr 2 O7, and pyridinium chlorochromate (PCC). Because all these Chromium reagents proceed through a mechanism which involves loss of a proton on the oxygen-bearing carbon of the alcohol, tertiary alcohols (which do not have such a hydrogen) are incapable of being oxidized by these reagents. Secondary alcohols are oxidized to ketones easily and cleanly. Primary alcohols are very easily oxidized by Cr(+6), but, if any water is present in the reaction, the product observed is a Carboxylic Acid rather than an aldehyde. Thus with aqueous reagents such as Jones'Reagent, the 1˚ alcohol undergoes overoxidation (unless we happen to wnt the acid). One solution is to use PCC in a nonaqueous medium. 17 R C R O OH H CrO3, H2SO4 Mechanism R C R R JONES REAGENT O Cr H O H O O R H3O+ R C Secondary alcohol is oxidized to a ketone with Jone's Reagent. R O H O Cr C O O H O R H O Cr C R H O OH O O R Cr + C R O O O R OH C + Cr R OH OH Alcohol must have hydrogen on the oxygen bearing carbon. Tertiary Alcohols will not undergo oxidation. Oxidation of 2˚ Alcohols to Ketones Oxidation of 1˚ alcohol with Jones' Reagent R OH CrO , H SO 3 2 4 C H H R O C H2O H OH R C H OH In presence of water the hydrate is formed which can undergo further oxidation Aldehyde is formed but none isolated CrO3 R O C OH Carboxylic acid is end product Oxidation of 1˚ alcohol with Pyridinium Chlorochromate (PCC) R C H H OH CrO HCl 3, (g) N R O C H Note that this reagent combination has no water, the aldehyde produced cannot undergo hydration. This is the preferred method for the synthesis of aldehydes, Oxidation of 1˚ Alcohols to Aldehydes with PCC e. Periodic Acid Cleavage of 1,2-Diols 1,2-Diols are oxidatively cleaved by aqueous periodic acid. This is a mild reaction and offers a useful alternative to cleavage with O3, which requires an expensive ozone generator and procedes through a dangerously explosive oxonide intermediate. 18 OH 1) OsO4 2)NaHSO3 OH H The periodic acid cleavage of C O 1,2-diols is an alternative to H the ozonolysis method for converting alkenes into carbonyl C O compounds. H5IO6 PERIODIC ACID 1,2-DIOL Mechanism OH OH HO OH - 2 H2O I + O HO OH OH Periodic Cleavage of 1,2-Diols. HO O HO I OH O O OHC (CH2) CHO + H3IO4 The periodic acid cleavage requires Cyclic intermediate the existance of a five membered intermediate. Diols that do not allow the existance of such an intermediate do not undergo reaction.







