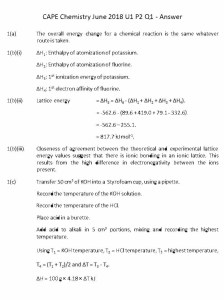
Chap 3 Solns-5/E

12-35
12.30 This problem asks that we compute the number of Frenkel defects per cubic meter in silver chloride at 350 ° C. Solution of this problem is possible using Equation 12.2. However, we must first determine the value of
N , the number of lattice sites per cubic meter, which is possible using a modified form of Equation 4.2; thus
N =
A
Ag
N
A
ρ
+ A
Cl
=
(6.023 × 10
23
atoms/mol)(5.50 g/cm
3
)(10
6
cm
3
/ m
3
)
107.87 g/mol + 35.45 g/mol
= 2.31 x 10
28
lattice sites/m
3
And, finally the value of N fr
is computed using Equation 12.2 as
N fr
= N exp
⎛
⎜
⎝
−
Q fr
2 kT
⎞
⎟
= (2.31 × 10
28
lattice sites/m
3
⎢
⎡
⎢
⎣ (2)(8.62 × 10
-5
1.1 eV
eV/K)(350 + 273 K)
⎤
⎥
⎥
= 8.24 x 10
23
defects/m
3
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for testing or instructional purposes only to students enrolled in courses for which the textbook has been adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.