Unit 2- Chemistry
advertisement

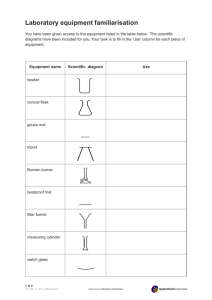
Unit 2 Chemistry Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases LESSON OUTLINE: I. II. III. IV. Matter a. Phases of Matter b. Conservation of Matter c. Physical and Chemical Change d. Phase Change and Energy e. Gas Laws Elements and the Periodic Table a. Atomic Models b. Periodic Table Organization c. Metals vs. Nonmetals Atoms and Bonding a. Chemical Symbols b. Chemical Formula Writing c. Chemical Bonding Chemical Reactions a. Balancing Equations b. Acids and Bases c. Neutralization of an Acid KEY CONCEPTS: As students progress through the year learning about the biotic and abiotic environment, the movement of chemicals in pathways, like Nitrogen and Sulfur in acid rain production, and cycles such as the carbon cycle, becomes very important. This unit is designed to give the students the “chemical language skills” needed to understand these pathways and cycles. OUTCOMES: Upon completion of this lesson, students will be able to: 1. describe how matter is conserved even though it may change properties as it forms compounds or changes physically. 2. explain how the periodic table is organized based on the atomic model and how the characteristics of different elements are related to the number of electrons on the outer shell of the element’s atoms. 3. write proper chemical formulas based on the atoms valence electrons in the compound. 4. describe the properties of acids and bases and describe with a chemical equation how an acid and a base can combine to make a salt and water. GRADUATION STANDARDS: The following MN graduation standards apply: 8.2.1.1.1 Distinguish between a mixture and a pure substance and use physical properties to separate mixtures and identify pure substances. 8.2.1.1.2 Use physical properties to distinguish between metals and non-metals. 8.2.1.2.1 Identify evidence of chemical changes, including color change, generation of a gas, solid formation and temperature change. 8.2.1.2.2 Distinguish between chemical and physical changes in matter. 8.2.1.2.3 Use the particle model of matter to explain how mass is conserved during physical and chemical changes in a closed system. 8.2.1.2.4 Recognize that acids are compounds whose properties include a sour taste, characteristic color changes with litmus and other acid/base indicators, and the tendency to react with bases to produce a salt and water. 9.2.1.2.1 Describe the role of valence electrons in the formation of chemical bonds. 9.2.1.2.2 Explain how the rearrangement of atoms in a chemical reaction illustrates the law of conservation of mass. 9.2.1.2.3 Describe a chemical reaction using words and symbolic equations. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases LEARNING ACTIVITIES: I. Assign reading: (6 hours) Chapter 1 sections 1-3 and Chapter 2 sections 1-3: from the Prentice Hall Science Explorer ©2009 Chemical Building Blocks and have students do reading previews, answer key concept questions, define key terms and do the Target Reading Skills. II. Phases of Matter: (1 hour) “What Changes Do You See” Introduce matter with a simple observation exercise of the different phases of matter. a. Materials i. Beaker ii. Watch plate iii. Ice cubes iv. Food coloring v. Hot water b. Procedure: Have the students follow the following procedure with ice, water, beaker. i. Fill a beaker 1/3 full of HOT water. ii. Put one drop of food coloring in the hot water iii. Place an ice cube in the hot water. iv. Cover the beaker with the watch glass. v. Place an ice cube on top of the watch glass. c. Have students answer the following questions as they observe the apparatus. i. What state of matter is the water? __________________ (Solid, Liquid, Gas) ii. What state of matter are the ice cubes? __________________ (Solid, Liquid, Gas) iii. What changes in the state of matter is occurring to the ice cube in the water? iv. What changes in the state of matter is occurring at the surface of the water? v. What changes in the state of matter is occurring on the bottom of the watch glass? vi. Why is the water on the bottom of the watch glass not colored? d. Conclusion: i. What determines the state of matter? ii. Review experiment questions at the end of the hour. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases III. Conservation of Matter: (1 hour) “What is Matter” Introduce the law of conservation of matter with the following exercise. Have the students describe the law by doing the following experiment. a. Notes: - Matter is anything that has mass and occupies space. b. Ask the Question: What happens to the mass of water when you add sugar? c. Materials: i. Water ii. Sugar iii. Triple-beam Balance d. Have students determine if the mass changes when combining sugar and water. i. Measure 100 ml of water ii. Find the mass of the water (don’t include the mass of the graduated cylinder) iii. Measure 10 g of sugar iv. Mix sugar and water v. Measure the final mass of water and sugar mixture e. Post lab questions: Ask students to answer the following questions. i. What was the starting mass of the water? ii. What was the mass of the sugar? iii. What happened to the mass of the water after you added sugar? iv. How close was the mass of the sugar plus the mass of the water to the mass of the combined sugar and water mass? Mass of sugar + Mass of water = Mass of combined water and sugar f. Conclude the last 15 minutes of the class period by having the students answer the question “A glass of unsweetened lemonade weighs 255 grams. A spoonful of sugar weighs 25 grams. Predict how much you think the sweetened lemonade will weigh after you stir in the sugar.” From the “Uncovering Student Ideas in Science: 25 formative assessment probes” Keeley, Page. Uncovering Student Ideas in Science. Arlington, VA: NSTA, 2005. Print. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases IV. V. Physical and Chemical Change Card Activity: (1 hour) Have the students use the cards in this activity to introduce examples of physical and chemical changes and have the students build a working definition of “physical” and “chemical” change. At the end of the class period, to fill out an exit ticket, “Define a chemical change.” And “Define a physical change.” Collect the exit tickets as the students leave the classroom. Use it as a tool to measure the growth in understanding from today’s lesson. Physical and Chemical Stations Lab: (1 hour) At the start of the hour review student responses to exit ticket. Then have students move between 7 stations (3 minutes per sation) to decide if the change happening at the station is a physical or chemical change based on the definition given in class at the start of the hour, and have them explain their reasoning. a. Materials i. Matches ii. Test tubes iii. Salt water bath iv. Vinegar v. Baking soda vi. Seltzer tablet vii. Ice viii. Potato ix. Iodine Solution x. Salt b. Procedure: Set up and have the students cycle through the following seven stations: i. Station 1 - Matches – chemical change – gas production, light, heat Strike a match and watch it as it burns. Blow the match out before it burns your fingers. When your observations are completed, rinse the used match in tap water and dispose of it in the watch glass on lab table. ii. Station 2 -Water Baths - physical change – phase change Fill a test tube ¼ or less with tap water. Place it in the salt-water ice bath and stir it around gently and constantly for 2-3 minutes (possibly more) without removing it. Take the test tube out. Record your observations. Rinse the inside AND outside of the test tube well with water and return to the test tube rack. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases VI. iii. Station 3 – Vinegar and baking soda – chemical – gas production Put about 1 cm of vinegar into a test tube. Then use a stick to put a SMALL (about ½ cm long on the stick) amount of baking soda into the tube. After your observations are complete, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. iv. Station 4 - Seltzer Tablet – chemical change gas production Fill a test tube about ¼ full with tap water. Put a small piece of crushed seltzer tablet into the water in the test tube. When you have completed your observations, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. v. Station 5 - Ice – physical change – phase change Put 2 or 3 small pieces of ice into a test tube and observe what happens as the solid ice turns to a liquid. When you have completed your observations, pour the test tube contents into the sink and rinse it before returning it back to the rack. vi. Station 6 Iodine on a Potato – chemical change – color change Cut a SMALL fresh slice from the potato. Use a dropper to put 34 drops of iodine solution on the potato slice. After your observations are complete, throw away the used slice of potato into the trashcan vii. Station 7 Salt – physical change – dissolving of a solid Fill a test tube about ¼ full with tap water. Use a spoon handle to add 1/2 cm (long on the stick) of salt to the water. Shake the test tube gently until the salt dissolves. When you have completed your observations, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. c. Conclusion: Based on the previous exercise, and today’s activity, ask if anything was created or destroyed, or were things changed from one form to another. Just because things changed, was there anything that stayed the same? Return the students answers the “lemonade probe” and discuss if the lab stations Chemistry in a Bag: (2 hours) This activity has students build on previous day’s activities, have students perform the following 3 lab exercises to observe chemical reactions and to observe changes in pH. Show an image of the pH scale and show, which side is acid and which is Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases base, and explain how an indicator can help us determine this. (www.middleschoolscience.com/bag.htm) a. Materials i. Baking soda ii. Phenolphthalein (or cabbage juice) iii. Test tube iv. Clear ziplock bags v. Calcium chloride b. Procedures: Have the students do the following three experiments: i. Procedure Bag 1: 1. Place 2 tsp of baking soda into the bag. 2. Add 30 mL of Phenolphthalein to the bag. (Skip this step if you are using cabbage juice) 3. •Massage the bag to mix the contents. 4. VERY CAREFULLY - lower the test tube containing 30 mL of water upright into the bag. (OR Lower a small cup or beaker with 50 ml of cabbage juice as a substitute). Do not let any spill out. 5. Have the student hold the test tube gently from the outside of the bag while squeezing the excess air out and seal the bag. 6. Hold the test tube and bag up and slowly spill the water out of the test tube (while the bag is still sealed). The contents will turn bright pink. Cabbage juice will turn greenish blue for a base and purple-ish for neutral, pink for acid. 7. As the students look at and feel the contents of the bag they will see the baking powder dissolving and the contents turning pinker. ii. Procedure Bag 2: 1. Place 2 tsp of calcium chloride into the bag. 2. Add 30 mL of Phenolphthalein to the bag. (Skip this step if you are using cabbage juice) 3. Massage the bag to mix the contents. 4. VERY CAREFULLY - lower the test tube containing 30 mL of water upright into the bag. (OR Lower a small cup or beaker with 50 ml of cabbage juice as a substitute) Do not let any spill out. 5. Have the student hold the test tube gently from the outside of the bag while squeezing the excess air out and seal the Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases bag. 6. Hold the test tube and bag up and slowly spill the water out of the test tube (while the bag is still sealed). The contents will turn bright pink and start to get warm. cabbage juice will turn greenish blue for a base and purple-ish for neutral, pink for acid. 7. As the students look at and feel the contents of the bag. They will see the calcium chloride dissolving and the contents turning pinker and getting warmer. iii. Procedure Bag 3: 1. Place 2 tsp of baking soda into the bag. 2. Place 2 tsp of calcium chloride. 3. Add 30 mL of Phenolphthalein. (Skip this step if you are using cabbage juice) 4. Massage the bag to mix the contents. 5. VERY CAREFULLY - lower the test tube containing 30 mL of water upright into the bag. (OR Lower a small cup or beaker with 50 ml of cabbage Juice as a substitute) Do not let any spill out. 6. Have the student hold the test tube gently from the outside of the bag while they squeeze the excess air out and seal the bag. 7. Hold the test tube and bag up and slowly spill the water out of the test tube (while the bag is still sealed). The contents will turn bright pink. Cabbage juice will turn greenish blue for a base and purple-ish for neutral, pink for acid 8. Have the students write down observations. At this point, they will see the baking powder and calcium chloride dissolving, foaming, bubbling, instead of pink, it will become clear/white, and the bag will be warm. The bag will start to inflate and puff out. c. Conclusions: Students should write a conclusion as to why each bag did reacted the way it did. Students should be able to identify each chemical and physical change that happened and what caused each of them. VII. Boil ice water: (2 hour) with temperature probes to graph the temperature change over time as energy is added to the system. This lab allows students to see how energy is needed to change the phase. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases a. Materials i. Beaker ii. Ice iii. Probeware/Thermometer iv. Hot plate b. Procedure i. Fill the beaker halfway with ice. Do not place the beaker on the hot plate yet. Observe the beaker of ice for 3-4 minutes. Draw a picture and record any changes in your notes. ii. Place the probeware or regular thermometer in the beaker so it is between the ice cubes. If you are using a regular thermometer, have the students make a data table in their notebook and record the beginning temperature on the data table at 0 min. If you are using the probeware, press "start." The program will record and graph the time temperature for the entire lab. Do not press stop until the water has reached a boiling point and has boiled for three minutes. iii. Place the beaker of ice on the hot plate and turn the dial to 7. If you are using a regular thermometer, use a stopwatch and record the temperature every minute in the data table. Continue to collect data until the water has begun to boil and has boiled for three minutes. If you are using the probeware, remember to continue to collect the data until the water has reached a boil and has boiled for three minutes. DO NOT PRESS "STOP." iv. After the water has boiled for three minutes, turn off the hot plate. Leave the beaker on the hot plate and allow it to cool. If you are using the regular thermometer, record your final reading. c. Conclusions: If the students are doing the data with a regular thermometer, have them graph their data. If they used the probeware have them use their graph and ask the students to compare their graph with the following images and go through these questions as a class. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases VIII. “It’s a Gas” Lab (1 hour) (Prentice Hall Science Explorer ©2009 Chemical Building Blocks) Have the students demonstrate the gas laws by applying pressure to and observing the air in a syringe. a. Materials i. Syringe ii. Clay iii. Books b. Procedure i. Make a data table in your notebook. ii. Lift the plunger of the syringe as high as it will move without going off scale. The volume inside the syringe will then be as large as possible. iii. Seal the small opening of the syringe with a piece of clay. The seal must be airtight. iv. Hold the syringe upright with the clay end on the table. With the help of a partner, place one book on top of the plunger. Steady the book carefully so it does not fall. v. With the book positioned on the plunger, read the volume shown by the plunger and record it in your data table. vi. Predict what will happen as more books are placed on top of the plunger. vii. Place another book on top of the first book resting on the plunger. Read the new volume and record it in your data table. viii. One by one, place each of the remaining books on top of the plunger. After you add each book, record the volume of the Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases syringe in your data table. ix. Predict what will happen as books are removed from the plunger one by one. x. Remove the books one at a time. Record the volume of the syringe in your data table after you remove each book. c. Analyze and Conclude: i. Graphing Make a line graph of the data obtained from Steps 5, 7, and 8. Show volume in cubic centimeters (cm3) on the vertical axis and number of books on the horizontal axis. Title this Graph 1. ii. Graphing Make a second line graph of the data obtained from Step 10. Title this Graph 2. iii. Predicting Did the results you obtained support your predictions in Steps 6 and 9? Explain. iv. Interpreting Data Compare Graph 2 with Graph 1. How can you explain any differences in the two graphs? v. Drawing Conclusions What does Graph 1 tell you about how the volume of a gas changes with increasing pressure? a. Communicating Write a paragraph explaining how the volume of the gas changed as books were added one by one. Base your explanation on what was happening to the gas particles in the syringe. IX. Assign reading: (5 hours) Chapter 3 sections 1- 5 from the Prentice Hall Science Explorer ©2009 Chemical Building Blocks and have students do reading previews, answer key concept questions, define key terms and do the Target Reading Skills. X. Reading jigsaw on the different atomic models, Dalton, Rutherford, Thompson, Bohr model: (1 hour) a. Have the students in 4 groups, assign each group a model to read about and have the students answer their assigned questions found on the following document. b. After the group has read and answered the questions, have them use the large portable white boards to draw the model, and put important characteristics of their atomic model and explain it to the rest of the class. As each group presents, to the rest of the class. XI. Periodic Table Basics Activity: (5 hours) After learning about the different atomic models, now the students can look at differences seen in the different elements known. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases a. Complete the squares for each element by adding the atomic number, name, and atomic mass. b. Determine the number of protons, neutrons, and electrons in each element. c. Create a Bohr diagram for each element. d. Draw the Lewis Structure for each element. e. Use the assigned colors to shade in the square for each element. You should ONLY color in the small square in the upper left-hand corner and not the entire card. f. Cut the cards apart and arrange according to atomic number in the pattern shown below. Once you have the cards arranged in the correct order, glue them to a large sheet of construction paper. XII. “Copper or Carbon – That is the Question” Lab: (1 hour) Prentice Hall Science Explorer ©2009 Chemical Building Blocks Have the students perform the following observations. Materials scientists work to find the best materials for different products. In this lab, students will look for an answer to the following problem: How do the properties of copper and graphite determine their uses? You will compare the properties of a copper wire and a pencil lead. Pencil lead is made mostly of graphite, a form of the nonmetal element carbon. a. Materials i. 250 mL beaker ii. Hot plate iii. Copper sample iv. Graphite sample v. Light bulb vi. Socket vii. Battery cell b. Procedure: i. Fill a 250-mL beaker about three-fourths full with water. Heat it slowly on a hot plate. Let the water continue to heat as you complete Part 1 and Part 2 of the investigation. ii. Part I - Physical Properties – 1. Compare the shininess and color of your copper and graphite samples. Record your observations. 2. Bend the copper wire as far as possible. Next, bend one of the graphite samples as far as possible. Record the results of each test. iii. Part II - Electrical Conductivity Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases 1. Place a bulb into a lamp socket. Use a piece of insulated wire to connect one pole of a dry cell battery to the socket. 2. Attach the end of a second piece of insulated wire to the other pole of the dry cell battery. Leave the other end of this wire free. 3. Attach the end of a third piece of insulated wire to the other pole of the lamp socket. Leave the other end of this wire free. 4. Touch the free ends of the insulated wire to the ends of the copper wire. Record your observations of the bulb. 5. Repeat Step 7 using a graphite sample instead of the copper wire. iv. Part III – Heat Conductivity 1. Turn off the hot plate. 2. Hold one end of a graphite sample between the fingertips of one hand. CAUTION: Be careful not to touch the beaker. 3. Dip both the graphite and copper wire into the hot water at the same time. Allow only about 1 cm of each piece to reach under the water’s surface. From your fingertips to the water, the lengths of both the graphite sample and the copper wire should be approximately equal. 4. Time how long it takes to feel the heat in the fingertips of each hand. Record your observations. c. Analyze and conclude using the following questions: i. Observing Compare the physical properties of copper and graphite that you observed. ii. Classifying: Based on the observations you made in this lab, explain why copper is classified as a metal. iii. Controlling Variables: In Step 11, why was it important to use equal lengths of copper wire and graphite? iv. Drawing Conclusions: Which of the two materials, graphite or copper, would work better to cover the handle of a frying pan? Explain your choice. v. Communicating: Write a paragraph explaining why copper is better than graphite for electrical wiring. Include supporting evidence from your observations in this lab. XIII. Alien Periodic Table Activity: (4 hours) Prentice Hall Science Explorer ©2009 Chemical Building Blocks Have the students imagine that Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases inhabitants of another planet send a message to Earth that contains information about 30 elements. However, the message contains different names and symbols for these elements than those used on Earth. a. Have the students figure out which elements on the periodic table do these “alien” names represent? i. The element doggone (D) has only 4 protons in its atoms. ii. Floxxit (Fx) is important in the chemistry of life. It forms compounds made of long chains of atoms. Rhaatrap (R) and Doadeer (Do) are metals in the fourth period, but rhaatrap is less reactive than Doadeer. iii. Magnificon (M), goldy (G), and sississ are all members of Group 15. iv. Goldy has fewer total electrons than magnificon. v. Urrp (Up), oz (Oz), and nuutye (Nu) all gain 2 electrons when they react. Nuutye is found as a diatomic molecule and has the same properties as a gas found in Earth’s atmosphere. Oz has a lower atomic number than urrp. vi. The element anatom (An) has atoms with a total of 49 electrons. vii. Zapper (Z) and pie (Pi) lose two electrons when they react. Zapper is used to make lightweight alloys. b. Analyze and conclude using the following questions. i. Drawing Conclusions: List the Earth names for the 30 alien elements in order of atomic number. ii. Classifying: Were you able to place some elements within the periodic table with just a single clue? Explain using examples. iii. Interpreting Data: Why did you need two or more clues to place other elements? Explain using examples. iv. Inferring: Why could you use clues about atomic mass to place elements, even though the table is now based on atomic numbers? c. Communicating: Write a paragraph describing which groups of elements are not included in the alien periodic table. Explain whether or not you think it is likely that an alien planet would lack these elements. XIV. Bonding Basics: (2 hours) Identify the number of valance electrons in a given atom, describe and demonstrate how an ionic bond forms, and describe and demonstrate how a covalent bond forms. Predict the type of bond that will occur between two elements. a. Write the symbols for each element. b. Use Skittles (or other cereal/candy with more than one color) to create Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases c. d. e. f. XV. the Lewis structure for each. Draw an arrow (or more if needed) to show the transfer of electrons and move the cereal to the new location. Determine the charge for each ion and write the formula. Make sure the sum of the oxidation numbers is zero and write the chemical formula. Have the students use a pencil or crayon to draw the electrons as they remove the pieces of cereal. Chemical Formula Writing: (3 hours) Using the knowledge that atoms bond the way they do because of their valence electrons, scientists can predict which atoms will combine together and make compounds that can be represented in chemical formulas. In their notebooks, have the students take notes, Chemical Nomenclature Notes formula writing.pdf. Then write the names or the formulas on the Chemical Formula Writing Assignment also found on the classroom website. XVI. Conductivity Lab Vernier: (2 hour) As students have used understanding of atomic structure to understand how atoms combine to make molecules or compounds, these compounds have different properties. In this experiment, students will study the electrical conductivity of water and various water solutions. A solution can contain molecules, ions, or both. Some substances, such as sucrose (C12H22O11), dissolve to give a solution containing mostly molecules. An equation representing the dissolving of sucrose (table sugar) in water is C12H22O11(s) ⎯⎯ → C12H22O11(aq) Other substances, such as calcium chloride (CaCl2), dissolve in water to produce a solution containing mostly ions. An equation is CaCl2(s) ⎯⎯ → Ca2+(aq) + 2 Cl-(aq) Have students use a Conductivity Probe to test the electrical conductivity of solutions, and determine which, molecules or ions, are responsible for electrical conductivity of the following solutions: sodium bromide, NaBr, solution potassium chloride, KCl, solution calcium chloride CaCl2, solution aluminum chloride, AlCl3, solution ethanol, C2H6O, solution ethylene glycol, C2H6O2, solution 2-propanol, C3H8O, solution Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases sucrose, C12H22O11, solution distilled water tap water a. Materials i. Conductivity probe and computer interface ii. sodium bromide, NaBr, solution iii. potassium chloride, KCl, solution iv. calcium chloride CaCl2, solution v. aluminum chloride, AlCl3, solution vi. ethanol, C2H6O, solution vii. ethylene glycol, C2H6O2, solution viii. 2-propanol, C3H8O, solution ix. sucrose, C12H22O11, solution x. distilled water xi. tap water b. Procedure i. Obtain and wear goggles. ii. Connect the Conductivity Probe to the computer interface. It should be set on the 0–20000 µS/cm position. Conductivity is measured in microsiemens per centimeter (µS/cm). iii. Prepare the computer for data collection by opening the file “16 Conducting Solutions” from the Physical Science with Vernier folder. iv. Measure the conductivity for each of the solutions. CAUTION: Many of these solutions are poisonous. Avoid inhaling any vapors from the solutions. Avoid contacting them with your skin or clothing. Be careful not to contaminate the solutions. v. Raise the beaker until the hole in the probe end is completely submerged in the solution as shown in Figure 1. Swirl the solution briefly. vi. Once the reading has stabilized, record the conductivity value. vii. Before testing the next solution, clean the probe by surrounding it with a 250 mL beaker and rinsing it with distilled water from a wash bottle. Carefully blot the probe dry with a tissue. c. Analyze and conclude with the following questions: i. Consider solutions 2–9. Which solutions conduct electricity best— those containing mostly ions or those containing mostly molecules? ii. Does distilled water conduct electricity well? Explain. iii. Does tap water conduct electricity? Account for this observation. iv. Consider the conductivity readings for the KCl, CaCl2, and AlCl3 solutions. What trend do you observe? Account for this trend. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases v. Suggest three other substances whose water solutions would conduct electricity well. Explain how you made your choices. d. EXTENSION i. Test your predictions for Question 5 above. XVII. Balancing Chemical Equations: (2 hours) Students have built understanding of atomic bonding, and understand that the law of conservation of mass applies to matter. Now when ionic compounds form, the ions come together the students will balance out the charges on the ions. The chemical formula for the compound reflects this balance. Have the students work on the Balancing Chemical Equations worksheet. XIII. pH Lab: (3 hours) During this activity students will recognize that acids are compounds whose properties include a sour taste, and characteristic color changes with litmus. And bases are compounds whose texture tends to be slippery, and have characteristic color changes with litmus. a. Materials i. Beakers ii. Mr. Clean liquid iii. Grapefruit Juice iv. Milk v. Vinegar vi. Soap water vii. Baking soda in water viii. Lemon juice b. Procedure: Students move between 7 stations to measure pH of the different liquids using pH paper, record the reaction of the blue and red litmus paper to the liquid, and depending on the liquid, describe the smell, feel and/or taste of the liquid. Students record this information on a post-it note for each liquid and at the end of visiting all 7 of the stations the students put the post-it notes on a large pH scale out in the hallway. Students look for similarities between the post-it notes on the scale of 1 to 14. i. Stations include: Mr. Clean liquid, grapefruit juice, milk, vinegar, soapy water, baking soda in water, lemon juice. c. Wrap-up: After the students have looked for patterns on the hallway pH scale, ask the following questions: i. What did you notice for substances that had a pH of less than 7? (sour taste) ii. What about substances that had pH of more than 7? (slippery texture) Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases iii. What could you say about an unknown substance’s pH if it felt slippery? (above 7) XIX. Neutralization of an Acid Lab: (2 hours) In a culminating activity, have the students mix equal amounts of an acid (.1M HCl) and a similar molar base (.1M NaOH) with an indicator that changes at 7 (phenolphthalein), and the tendency to react with bases to produce a salt and water. a. Use the procedure from Long Beach High School. b. Analyze and Conclude: In the last 15 minutes of the class period, ask the students the following questions (exit ticket if you have time): i. On average how much of the base was needed to change the 10 mL of acid to a neutral solution (where the phenolphthalein indicator changed color pH of 7)? ii. What are the products of mixing an acid with a base? iii. Finish the equation HCl + KOH à ??? XX. Antacid Test: (2 hours) Prentice Hall Science Explorer ©2009 Chemical Building Blocks The students at this point have the understanding of how chemicals behave and how they can be observed. The students will focus on the following skills; designing experiments, interpreting data, and measuring. Which antacid neutralizes stomach acid with the smallest number of drops? a. Materials i. Hydrochloric acid ii. Plastic dropper iii. Different antacid iv. Methyl orange solution b. Procedure: i. Part 1 1. Using a plastic dropper, put 10 drops of hydrochloric acid (HCl) into one cup. CAUTION HCl is corrosive. Rinse spills and splashes immediately with water. 2. Use another plastic dropper to put 10 drops of liquid antacid into another cup. 3. In your notebook, make a data table to record the colors of the HCl and the antacid. 4. Add 2 drops of methyl orange solution to each cup. Record the colors you see. 5. Test each of the other antacids. Discard all the solutions and cups as directed by your teacher. ii. Part 2 Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases 1. Methyl orange changes color at a pH of about 4. Predict the color of the solution you expect to see when an antacid is added to a mixture of methyl orange and HCl. 2. Design a procedure for testing the reaction of each antacid with HCl. Decide how many drops of acid and methyl orange you need to use each time. 3. Devise a plan for adding the antacid so that you can detect when a change occurs. Decide how much antacid to add each time and how to mix the solutions to be sure the indicator is giving accurate results. 4. Make a second data table to record your observations. 5. Carry out your procedure and record your results. 6. Discard the solutions and cups as directed by your teacher. Rinse the plastic droppers thoroughly. c. Analyze and Conclude: i. Designing Experiments: What is the function of the methyl orange solution? ii. Interpreting Data: Do your observations support your predictions from Step 6? Explain why or why not. iii. Inferring: Why do you think antacids reduce stomach acid? Explain your answer, using the observations you made. iv. Controlling Variables: Explain why it is important to use the same number of drops of HCl in each trial. v. Measuring: Which antacid neutralized the HCl with the smallest number of drops? Give a possible explanation for the difference. vi. Calculating: If you have the same volume (number of drops) of each antacid, which one can neutralize the most acid? vii. Drawing Conclusions: Did your procedure give results from which you could draw conclusions about which brand of antacid was most effective? Explain why or why not. d. Design an Experiment: (if time permits) A company that sells a liquid antacid claims that its product works faster than tablets to neutralize stomach acid. Design an experiment to compare how quickly liquid antacids and chewable antacid tablets neutralize hydrochloric acid. Obtain your teacher’s permission before carrying out your investigation. Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases RESOURCES: Prentice Hall Science Explorer 2009 “Chemical Building Blocks” Lemonade worksheet https://primaryproficient.wikispaces.co m/file/view/Lemonade.pdf E2 Class website https://murray.spps.org/e2_environme ntal_inquiry_immersion_2.html Physical and Chemical Change Lab http://www.slcschools.org/department s/curriculum/science/Grade-7-to8/Grade-8/documents/Physical-andChemical-Changes.pdf Famous Scientist Worksheet http://www.learnnc.org/lp/multimedia /11342 Periodic Table Basics http://www.sciencespot.net/Media/pta blebasics.pdf Chemical Bonding Basics http://sciencespot.net/Media/bonding basics2010.pdf Balancing Chemical Equations Worksheet http://www.kentchemistry.com/ Worksheets/Regents/Units/Math ofChem/WSBalancing21.pdf Acid Base Titration Lab www.kentchemistry.com/Labs/Lab%20 Titration.doc EQUIPMENT: Phases of Matter • Beaker • Watch plate • Ice cubes • Food coloring • Hot water Conservation of Matter • Water • Sugar • Triple-beam Balance Physical and Chemical Stations • Matches • Test tubes • Salt water bath • Vinegar • Baking soda • Seltzer tablet • Ice • Potato • Iodine Solution • Salt Chemistry in a Bag • Baking soda • Phenolphthalein (or cabbage juice) • Test tube • Clear ziplock bags • Calcium chloride Boil Ice Water • Beaker • Ice • Probeware/Thermometer • Hot plate It’s a Gas Lab • Syringe • Clay • Books Copper or Carbon • 250 mL beaker • Hot plate • Copper sample • Graphite sample • Light bulb • Socket • Battery cell Conductivity Lab Unit 2- Chemistry: Matter/Periodic Table/Acids & Bases Conductivity probe and computer interface • sodium bromide, NaBr, solution • potassium chloride, KCl, solution • calcium chloride CaCl2, solution • aluminum chloride, AlCl3, solution • ethanol, C2H6O, solution • ethylene glycol, C2H6O2, solution • 2-propanol, C3H8O, solution • sucrose, C12H22O11, solution • distilled water • tap water pH Lab • Beakers • Mr. Clean liquid • Grapefruit Juice • Milk • Vinegar • Soap water • Baking soda in water • Lemon juice Antacid Test • Hydrochloric acid • Plastic dropper • Different antacid • Methyl orange solution • GLOSSARY: Matter - Anything that has mass and occupies space. Chemistry - The study of the properties of matter and how matter changes. Physical property - A characteristic of a substance that can be observed without changing the substance into another substance. Chemical property - A characteristic of a substance that describes its ability to change into different substances. Element - A substance that cannot be broken down into any other substances by chemical or physical means. Atom - The smallest particle of an element. Chemical bond - The force that holds atoms together. Molecule - A neutral particle made of two or more atoms joined by covalent bonds. Compound - A substance made of two or more elements chemically combined in a specific ratio, or proportion. Chemical formula - A combination of symbols that represents the elements in a compound. Physical change - A change that alters the form or appearance of a material but does not make the material into another substance. Chemical change - The process in which substances undergo chemical changes that result in the formation of new substances. Law of conservation of mass - The principle stating that matter is not created or destroyed during a chemical reaction.