I. TITLE: Flame Emission Spectroscopy II. OBJECTIVE
advertisement

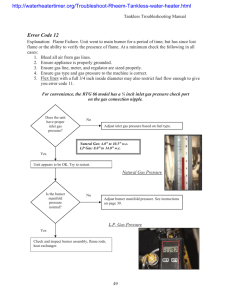
Name___________________________________ Per. __________ I. TITLE: Flame Emission Spectroscopy II. OBJECTIVE: Students should understand that when various elements and compounds are heated, they give off unique wavelengths of light which can be used to identify them. The student should also be able to explain how this can be used to learn about the chemical composition of stars. III. PROCEDURES: Much of what we know about the universe we have learned by making visual observations. Although this was originally limited to nearby objects, modern technology has allowed detailed images from all over the universe to be captured and studied. Scientists found they could match the visible light spectra of stars to chemicals found right here on earth by heating those chemicals in a lab. The spectral colors that are given off by a star can tell us about its temperature, chemical makeup, relative speed, density, etc. Today you will learn how to recognize several atom's "fingerprints" in the normal light spectrum. All matter is made up of atoms. Surrounding the nucleus of an atom are shells of electrons - small negatively charged particles. These shells are actually different energy levels and within the energy levels, the electrons orbit the nucleus of the atom. When an electron absorbs too much energy, it jumps away from the nucleus into a new shell or orbit. To return to its original orbit, the electron must give off a photon of light which is what we will see! 1. Each station is self-contained with chemical, splints, burner, and striker, or a spectroscope and light source 2. Start at one of the six chemical stations or three light stations with spectroscopes; if chemical record it in Table 1.You will rotate clockwise until you have completed all nine stations. 3. Light the burner by turning the gas jet on so that the lever is parallel with the jet, hold the striker tilted and above the burner and give it 5 clicks, if it doesn't light, shut off the gas before trying again. 4. Dip the splint wetted with distilled water into the edge of the chemical in the paper cup and place the splint/chemical briefly over the flame. DO NOT light the splint on fire. 5. The chemical should now give off a colored flame; remove from flame and dunk into used splint beaker. Turn the burner off with the handle. Record the color on Table 1 next to the chemical. 6. Refer to Figure 1 or the front screen to find out the wavelength of the light you saw for each chemical; record this information on Table 1. Discus, and come to a group consensus. 7. For visible light stations, observe either the ceiling, incandescent, or natural light and draw the colors of the spectrum observed over the visible light grid 8. Tidy up your last station and return to your seat. IV. OBSERVATIONS: Table 1 COMPOUND Figure 1: WAVELENGTH (nm) COLOR SEEN Ceiling (fluorescent) light: 750 650 550 450 750 650 550 450 750 650 550 450 Incandescent Light Sunlight Compare and contrast the three observed spectra. V. INTERPRETATIONS: 1. Which atoms in the chemical compounds, do you think, are responsible for the production of the colored light? 2. Why do different chemicals emit different colors of light? 3. Why do you think the chemicals have to be heated in the flame first before the colored light is emitted? 4. Where else have you observed colorful light emissions in everyday life? 5. Did any of the chemicals you tested produce similar results? Why do you think this happened? 6. Why were the splints soaked in distilled water prior to use? VI. CONCLUSION: If a star was observed to have nearly identical colorful emission lines to our sun, but it was slightly more blue in color, what could you tell about the star?