MSE 440/540 Test 1 Spring 2012 104 Points Total
advertisement

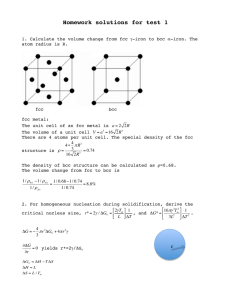
MSE 440/540 Test 1 Spring 2012 104 Points Total Name(print)______________________________ Undergraduate Or Graduate (circle one) ID number _______________________________ No notes, books, or information stored in calculator memories may be used. The NCSU academic integrity policies apply to this exam. As such, by taking this exam you are implicitly agreeing with the statement: “I have neither given nor received unauthorized aid on this test.” All work must be written on these pages and turned in. To receive full or partial credit on numerical problems, you must show your calculations in step-by-step fashion. Units must be shown when applicable and plots must have labeled axes. Be sure that you read and answer all parts of each question. Constants, equations, and data are given at the end of the exam. 1-3.) (4 Points Each-12 Points Total) Multiple Choice/Filling Blanks 1. When a metal changes crystal structure in the solid state at some critical temperature, this is known as a [ phase ] transformation. 2. Heterogenous nucleation into fine grains is enhanced through the use of [ seeds ]. 3. Compared with homogeneous nucleation, heterogeneous nucleation has a) the same energy barrier b) the same critical nucleus radius 4. (15/10 points U/G) Calculate the volume change from fcc γ-iron to bcc α-iron for unit cells. The atom radius is R. An fcc unit cell contain 4 atoms. The unit cell edge length a = 2R21/2 The unit cell volume cell V fcc = a 3 = (2 2R)3 = 16 2R 3 The volume of each atom is: atom cell V fcc = V fcc / 4 = 4 2R 3 A bcc unite cell contain two atoms. The unit cell edge length = 4R/31/2 The unit cell volume cell Vbcc = a 3 = (4R The volume of each atom is: 3)3 = 64R 3 3 3 atom cell Vbcc = Vbcc / 2 = 32R 3 3 3 atom atom Vbcc −V fcc 32R 3 3 3 − 4 2R 3 ×100% = = 8.87% The volume change = atom V fcc 4 2R 3 5. (6 points) List the effects of increasing undercooling • Higher nucleation rate leads to finer grain size • Higher driving force for nucleation and growth • Faster solidification • May leads to negative temperature gradient at very large undercooling • More limited defusion 1 6. (20 points) Describe the microstructures of gray, white, malleable, and ductile cast irons. Discuss how each is formed in terms of solidification behavior, composition, and heat treatment. See the ppt for lecture 6 7. (15/10 points, U/G) Why do metals not solidify exactly at the melting temperature, that is, where the Gibbs free energies of the liquid and solid phases are equal? What determines the range of temperature over which metals are observed to solidify? For an alloy: a) A negative Gibbs free energy change ΔG is required to drive the solidification. At the melting temperature, the driving force ΔG is zero. b) 1) the equilibrium starting T and ending T on phase diagram. The starting solidification T is slightly lower than the equilibrium starting T. The ending solidification T is below the equilibrium ending T. The deviation depends on the cooling rate. Larger cooling rate results in larger deviation. For a pure metal or an alloy with a single melting point (e.g. eutectic alloy): The solidification occurs at a temperature that is slightly below the melting point. 8. (10 points) What properties determine the quality of a sand mold for sand casting? • • • • • Strength - to maintain shape and resist erosion Permeability - to allow hot air and gases to pass through voids in sand Thermal stability - to resist cracking on contact with molten metal Collapsibility - ability to give way and allow casting to shrink without cracking the casting Reusability - can sand from broken mold be reused to make other molds? 9. (10 points) Define 1) Planar growth, 2) Dendritic growth See Page 11 (or12) of Lecture 2. 10. (6 points) What mold property will be affected most by the grain size of sand and how? Large grain size produce better permeability, but rougher cost surface. 2 11. (10 points) In the casting of steel under certain mold conditions, the mold constant in Chvorinov's rule is known to be 4.0 min/cm2, based on previous experience. The casting is a flat plate whose length = 30 cm, width = 10 cm, and thickness = 20 mm. Determine how long it will take for the casting to solidify. Solution: Volume V = 30 x 10 x 2 = 600 cm3 Area A = 2(30 x 10 + 30 x 2 + 10 x 2) = 760 cm2 Chvorinov’s rule: TTS = Cm (V/A)2 = 4(600/760)2 = 2.49 min. 12. [14 points, GRAD ONLY] Label each of the following situations on the free energy curves. Give a short description of the likelihood of nucleation and growth in each case, and in terms of the competition between volumetric and surface free energy of a growing nuclei. 1) No undercooling 2) Small undercooling ----------ΔG(T1) 3) Moderate undercooling ----------------------ΔG(T2) 4) Large undercooling ----------ΔG(T3) Constants and Equations NA = 6.02 x 1023 mole-1 k (or R) = 8.62 x 10-5 eV/atom-K (or 8.31 J/mol•K) K = C +273 109 nm = 1 m γSL + γLV < γSV ΔS = ΔH m L = Tm Tm C.E.(%) = C% + G = H – TS ΔG ≅ 102 cm = 1 m H=U+PV LΔT Tm TTS ⎛ V ⎞ = Cm ⎜ ⎟ ⎝ A ⎠ n Si% + P% 3 3 €