Diffusion and Osmosis Lab APBiology Period n
advertisement

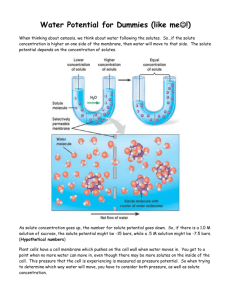
Diffusion and Osmosis Lab APBiology Period n NAME____________________________________ Abstract: In this lab we studied the movement of water across the cell membranes of potatoes in an effort to determine the effective water potential of potato cell cytoplasm. Potatoes cubes or cored cylinders were placed in sucrose solutions varying in concentration from 0 to 1 molar and allowed to reach dynamic equilibrium. Since sucrose cannot cross the plasma membrane, dynamic equilibrium was reached by osmosis, or diffusion of water across the semipermeable plasma membrane. Potato sections were massed, bathed in solution overnight, and massed again. We found that potatoes in 0 and 0.2 M sucrose gained mass, while those in 0.4 to 1 M sucrose lost mass. Our results indicate that solutions below and including 0.2 M sucrose are hypotonic, while solutions with sucrose concentrations 0.4M and higher were hypertonic. The isosmotic point lies in between the transition from hypotonic to hypertonic. Thus, we estimate that the osmolarity of potato cell cytoplasm is approximated by 0.3 M sucrose. Introduction: The kinetic energy of solutions causes molecules to move around and bump into each other. Diffusion is one result of this molecular movement, and results from random movement of molecules from an area of higher concentration to areas of lower concentration. Osmosis is a special kind of diffusion where water moves through a selectively permeable membrane (a membrane that only allows certain molecules to diffuse though). Diffusion or osmosis occurs until dynamic equilibrium has been reached. This is the point where the concentrations in both areas are equal and no net movement will occur from one area to another. There are three classifications of solutions based on their relative solute concentrations. If two solutions have the same solute concentration, the solutions are said to be isotonic. If the solutions differ in concentration, the area with the higher solute concentration is hypertonic and the area with the lower solute concentration is hypotonic. Since a hypertonic solution contains a higher level of solute, it has a high solute potential and low water potential. This is because water potential and solute potential are inversely proportional. A hypotonic solution would have a high water potential and a low solute potential. An isotonic solution would have equal solute and water potentials. Water potential (ψ) is composed of two main things, a physical pressure component, pressure potential (ψp), and the effects of solutes, solute potential (ψs). A formula to show this relationship is ψ = ψp - ψs. Water will always move from areas of high water potential to areas of low water potential. The solute potential ψs = i MRT, where , M = molarity and T = absolute temperature in Kelvin. i is the van 't Hoff factor, which depends on the manner in which a solute dissolves. i = 1 for nonelectrolytes, but it is approximately equal to the number of ions generated during dissolution for electrolytes. For example i for NaCl is 2, but for sucrose, i = 1. In this study we wished to determine the effective osmolarity of potato cell cytoplasm. In order to do so we placed potato pieces in solutions ranging from 0 to 1 M sucrose overnight and examined their change in mass. Since sucrose cannot likely cross the plasma membrane of the potato cells, the volume, and hence mass of the potatoes will change to reflect water movement required to reach dynamic equilibrium. We hypothesized that the osmolarity of potato cell cytoplasm is equivalent to a sucrose concentration between 0 and 1 M, and that some solutions would be hypertonic while others would be hypotonic. We indeed found that solutions could be grouped accordingly. Analysis of the results showed that there was a transition from hypotonic to hypertonic as sucrose concentration was increased from 0.2 M to 0.4 M, indicating that the isotonic point lay in between these two values. Materials and Methods: Potatoes were sliced into 1 cm3 pieces and massed on a 3 beam balance. Four pieces were massed together and placed into a 250 ml beaker containing ~50 ml of the test sucrose solution. Twenty four pieces in all were placed in six beakers containing 0 (tap water), 0.2, 0.4, 0.6, 0.8 and 1 M sucrose. After overnight incubation, the potato pieces were removed then lightly blotted dry and massed again. Percent changes in mass were calculated and data was combined from 6 groups to find the average change for each sucrose solution. We expected potato cell membranes to be impermeable to sucrose; thus, changes in mass likely reflected changes in water content. Lower masses after incubation indicated that water left the cytoplasm due to bathing in a hypertonic solution. Higher masses indicate water entering the cytoplasm due to bathing in a hypotonic solution. Results: First we present data from our group and then combined data from all groups in both AP classes. In Table 1, the original masses of each of the six sets of 4 potato pieces were recorded. The masses of the pieces after overnight incubation are also shown. The percent change was calculated as 𝑃𝑒𝑟𝑐𝑒𝑛𝑡 𝐶ℎ𝑎𝑛𝑔𝑒 = Table 1. Water 10.9 g 16 g 5.1 g 46.8% massorig massafter Change % change 0.2 8g 8.5 g 0.5 g 6.25 % 0.4 14 g 12 g -2 g -4.3 % 𝑚𝑎𝑠𝑠𝑎𝑓𝑡𝑒𝑟 − 𝑚𝑎𝑠𝑠𝑜𝑟𝑖𝑔 𝑚𝑎𝑠𝑠𝑜𝑟𝑖𝑔 0.6 9g 7.4 g -1.6 g -17.8 % 0.8 8g 6g -2 g 25 % 1.0 12.5 g 9.5 g -3 g 24 % Positive percent changes in mass indicate the potatoes were bathed in a hypotonic solution. Negative percent changes in mass indicate the potatoes were bathed in a hypotonic solution. Note the transition from positive to negative percent changes between 0.2 M and 0.4 M sucrose. We also present data from 9 groups across two AP biology classes in Table 2. Most groups recorded results similar to ours. Again, there was transition from positive to negative percent changes between 0.2 M and 0.4 M sucrose. Table 2. Column1 0 Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 1 Group 2 Group 3 Mean 0.2 15.09 0 23.1 46.8 23.74 13 15 21.4 6.89 0 13.4 6.25 2.54 -9.3 9 38 11 19.76625 8.642222 0.4 0.6 0.8 1 -10.28 -23.08 -30.77 -27.27 -3 -6 -11 -3 -5.8 -10.8 -9.1 -15.4 -14.3 -17.8 -25 -24 -15.38 -23.52 -36.25 -32.24 -13 -20.9 -27.7 -34 -16 -26 -34.5 -28 -15 -24 -30 -28.5 -9 -16 -29 -22 11.3067 18.6778 25.9244 23.8233 When we examine plots of the percent change vs. sucrose concentration for all groups (Figure 1). The transition from hypotonic to hypertonic occurs between 0.2 and 0.4 M, and the average line for all groups’ data crosses the line corresponding to zero percent change at approximately 0.3 M. Figure 1. 60 50 40 Series1 Series2 30 Series3 Series4 20 Series5 10 Series6 Series7 0 0 -10 -20 -30 0.2 0.4 0.6 0.8 1 Series8 Series9 Series10 Series11 MEAN VALUES -40 -50 Discussion: In this study we examined the effects of overnight incubation of potato pieces in varying concentrations of sucrose to determine the concentration of sucrose that most closely matches the water potential of the potato cell cytoplasm. Loss or gain of water was measured as changes in mass. We felt confident that the changes in mass reflected water movement rather than solute movement because potato cells are filled with starch and sucrose is a large molecule for which no cell membrane transporters have been identified. The solutions that were hypotonic to potato cell cytoplasm were the 0 and 0.2 M sucrose, whereas solutions of 0.4 M sucrose and greater were hypertonic. The transition from hypotonic to hypertonic occurred between 0.2 M and 0.4 M. Since the water potentials of the six solutions were 0, -496, -991, -1487, -1982, and -2478 N. Thus the water potential of potato cell cytoplasm lies between -496 and -991 N. Judging by the plots in Figure 1, linear interpolation between these the two points for 0.2 and 0.4 M sucrose gives a potato cell cytoplasm water potential of -743 N, which is equivalent to 0.3 M sucrose. Figure 1 also demonstrates that potato cells can absorb more water than they can release. Since the concentration dependence of percent mass changes do not fall on a straight line, but tends to diminish with increasing sucrose concentration, one could argue that the potatoes used in this study did not exhibit the maximum turgor pressure until placed in distilled water. For future study, perhaps potatoes could be incubated for varying durations to determine the time course of osmotic flow.