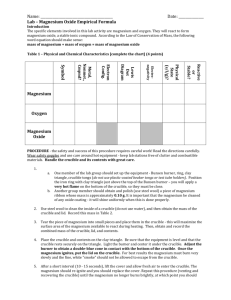
Lab One: Formation of a main group ionic compound Question: If
advertisement

Lab One: Formation of a main group ionic compound Question: If you react a main group metal and a non-metal, what compound will you make? Materials: Magnesium Ribbon Crucible Ring Stand Ring Clay Triangle Bunsen Burner Ruler Procedure (part 1): 1. 2. 3. 4. 5. 6. 7. Measure out approximately 2 centimeters of magnesium ribbon. Cut it with the scissors. Weigh your empty crucible. Place the magnesium ribbon in the crucible. Weigh it again. Set up your ring stand and clay triangle as demonstrated by your instructor. Heat your magnesium under intense flame for 3 minutes. Allow the crucible to cool for 5 minutes. Weigh you crucible again. Data Sheet: Label Data Empty Crucible Crucible + Mg Crucible + Product Change in Mass Procedure (part 2): 1. Add 10 drops of water to the solid in your crucible. 2. Warm slowly over the heat until the precipitate is dry (be very careful not to allow any splattering to occur). 3. Heat strongly for another 3 minutes. 4. Allow to cool for 5 minutes. 5. Reweigh your crucible again. Label Empty Crucible Crucible + Mg Crucible + Product (after part 2) Change in Mass Data 1. Using the data collected above in Part 1, determine the empirical formula of the compound you have made. 2. Is this formula close enough to the actual formula of the compound to be acceptable? Explain 3. Using the data collected above in Part 2, determine the empirical formula of the compound you have made. 4. Is this formula CLOSER to the actual formula of the compound? 5. In truth, when magnesium is heated in air, most of it reacts with oxygen gas, but some reacts with nitrogen gas to form magnesium nitride. Write the balanced chemical equation for the reaction between magnesium and nitrogen to form magnesium nitride. 6. Heating the magnesium nitride in water creates ammonia (NH3) and magnesium hydroxide. Write a balanced chemical equation for this reaction. 7. Magnesium hydroxide is unstable at high temperatures and decomposes into water and magnesium oxide. Write a balanced chemical equation for this reaction. 8. Why did we perform part two of the procedure? Explain using at least 3 complete sentences. 9. Using your empirical formula from part 1 of the lab, determine the ratio of Mg3N2 to MgO present in your compound from part 1. Is this ratio small enough to ignore? Support your answer using data.