60
The Golgi apparatus at the cell centre
Rosa M Rios and Michel Bornensy
In non-polarised mammalian cells, the Golgi apparatus is
localised around the centrosome and actively maintained
there. Microtubules and molecular motor activity are required
for determining both the localisation and organisation of the
Golgi apparatus. Other factors, however, also appear
necessary for regulating both the static steady-state
distribution of this organelle and its relationship with
microtubule minus-end-anchoring activities of the centrosome.
Several non-motor microtubule-binding proteins have now
been found to be associated with the Golgi apparatus. Recent
advances suggest that, in addition to important roles in cell
motility, polarisation and differentiation, the interplay between
Golgi apparatus and centrosome could participate in other
physiological processes such as intracellular signalling, mitosis
and apoptosis.
Addresses
Departamento de MicrobiologõÂa, Facultad de BiologõÂa, Universidad de
Sevilla, Reina Mercedes 6, 41012-Sevilla, Spain
e-mail: rmrios@us.es
y
Institut Curie, UMR144 du CNRS, 26 Rue d'Ulm, 75248 Paris, Cedex 05,
France
e-mail: mbornens@curie.fr
Current Opinion in Cell Biology 2003, 15:60±66
This review comes from a themed issue on
Cell structure and dynamics
Edited by Michel Bornens and Laura M Machesky
0955-0674/03/$ ± see front matter
ß 2003 Elsevier Science Ltd. All rights reserved.
DOI 10.1016/S0955-0674(02)00013-3
Abbreviations
BFA
brefeldin A
GA
Golgi apparatus
GAP
GTPase-activating protein
MT
microtubule
NZ
nocodazole
PKA
protein kinase A
c-TuRC g-tubulin ring complex
Introduction
Microtubules (MTs) play an essential role in membrane
traf®c processes in higher eukaryotic cells. MTs facilitate
membrane traf®c pathways connecting different organelles that also use the polarised microtubular array to
acquire de®ned subcellular positions. Among all membrane organelles, the Golgi apparatus (GA) of mammalian
cells is particular in that not only its subcellular localisation at the cell centre but its very existence as a single
organelle is dependent on MTs [1].
Current Opinion in Cell Biology 2003, 15:60±66
It is now widely accepted that the GA is in dynamic
equilibrium with the endoplasmic reticulum (ER). The
cargo exiting the ER is selectively packaged into pre-Golgi
transport intermediates that are subsequently translocated
along the MTs toward the centrosome powered by minusend-directed motors. There, these pre-Golgi elements,
thought to be the direct precursors of the GA, fuse to
generate the ®rst Golgi cisternae that, according to the
most widely accepted model, then mature from a cis (entry,
i.e. closest to the ER) to a trans (exit) form. Implicit in this
scheme is that biogenesis of the GA occurs around the
centrosome. Simultaneously, other membrane tubules and
vesicles form from the Golgi cisternae and are transported
back to the ER [2,3]. When these membrane traf®c pathways are interrupted, the organelle structure is altered,
supporting the idea that the maintenance of a single
central GA requires an appropriate balance of membrane
in¯ow and out¯ow pathways [2,4]. In this dynamic view,
molecular motors have arisen as the main actors in maintaining the organisation and positioning of the GA, and a
major focus of works in this area has been to clarify their
role in regulating membrane traf®c pathways [4].
Several important questions remain unanswered, however. How does the GA exhibit a de®ned steady-state
distribution within the cell and appears almost stationary
around the centrosome in spite of an enormous capacity
for dynamics and motility? How do MTs participate in
determining the overall three-dimensional arrangement
of the GA? In this review, we will discuss progress on
these questions, focusing on the relationship between
GA, MT minus ends and centrosome. New insights on
motor proteins will not be discussed, although, in addition
to cytoplasmic dynein 1 complex, two other minus-end
directed motors have been implicated recently in Golgi
positioning [5,6].
Structural association of the Golgi
apparatus and microtubules
Although three-dimensional electron microscopy studies
have advanced our understanding of the organisation of
the GA [7], the structural associations between Golgi
membranes and MTs has proven dif®cult to assess using
conventional methods. In a recent study, Marsh et al. [8]
used high-pressure freezing, freeze-substitution and electron tomography to study the three-dimensional structure
of the Golgi and surrounding organelles. These combined
techniques have allowed the authors to model individual
MTs and analyse their in situ relationship with organelles
in the Golgi region. The organisation of MTs in the
analysed region was similar to that of interphase epithelial
cells, and MTs did not seem to grow from the centrosome.
www.current-opinion.com
The Golgi apparatus at the cell centre Rios and Bornens 61
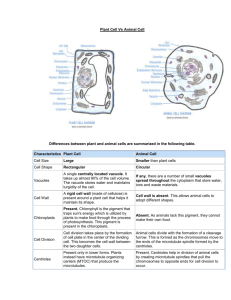
Figure 1
Three-dimensional reconstruction of a part of the Golgi ribbon, revealing in situ physical relationships between the cis-most cisternae and MTs. (a)
MTs (green) closely follow and occasionally form contacts with the membranes of the cis-most cisternae (blue). Note that in the modelled region,
MTs do not exhibit a typical radial organisation. Bar 500 nm. (b) A higher-magnification view oriented to show that the paths of some MTs closely
follow the membranes over considerable distances. MTs traversing the Golgi stack can be also observed. Images are reproduced with
permission from [8] (Copyright ß 2001, National Academy of Sciences USA).
Interactions between the Golgi stacks and MTs were
found to occur essentially at the cis face (Figure 1a). The
paths of individual MTs appeared to closely follow the
membranes of the ®rst cisternae (separated by 30 nm)
and occasionally make contact with it. In addition, MTs
traversing the Golgi stacks via cisternal openings at multiple points were observed (Figure 1b). Medial and trans
cisternae were not found to be associated with MTs.
These data support a speci®c role of MTs in the cis face
of the GA where new cisternae are generated. Maturation
of cis to trans cisternae would therefore implicate also the
relocalisation of MT-binding activities associated to the
cis-Golgi membranes, either by membrane recycling or by
association/dissociation from a cytoplasmic pool.
Consistent with these ®ndings, both motors [4,9] and nonmotor MT-binding proteins have been found to accumulate essentially at the cis face of the GA. GMAP-210 [10] is
a peripheral membrane protein that behaves like the
Golgi matrix proteins in response to brefeldin A (BFA),
a GA-disrupting agent. It mediates interactions between
Golgi membranes and stable MTs, and binds to MTs via
its carboxy-terminal domain, exhibiting a preference for
www.current-opinion.com
MT minus ends. Its overexpression induces the loss of
the MT aster, and the formation of a dense network of
short MTs that co-localises with the GA, suggesting a
MT-anchoring/stabilising activity for this protein. Under
these conditions of GMAP-210 overexpression, membrane transport from and to the GA is blocked [11] and
Golgi morphology and size are profoundly perturbed.
These data point to a role for this protein in the biogenesis
of the GA around the centrosome.
Hook proteins are MT-binding proteins that have been
proposed to link Golgi membrane organelles to MTs [12].
Among them, Hook3 has been proposed to play a role in
the localisation of the GA near the centrosome. Hook3
exhibits a cell-cycle-dependent localisation, and during
interphase, it is mainly detected in a juxtanuclear position
close to the centrosome. This localisation is MT-dependent and mostly insensitive to BFA. During prophase,
Hook3 accumulates at mitotic poles. A fraction of Hook3
was shown to be associated with cis-Golgi membranes and
to redistribute to peripheral sites after BFA treatment. In
addition, Hook3 overexpression induces fragmentation
and dispersion of the GA.
Current Opinion in Cell Biology 2003, 15:60±66
62 Cell structure and dynamics
Marsh et al. [8] also provided strong evidence for speci®c
association between the MTs and some tubulovesicular
elements broadly classi®ed as `endo±lysosomal compartments'. Moreover, a new cytoplasmic linker protein
(CLIP) from the CLIP-170 family, CLIPR-59, has been
shown recently to be associated with membranes of the
trans-Golgi network (TGN) and is proposed to play a role
in TGN±endosome dynamics [13].
Another interesting aspect is the relationship between the
GA and a subpopulation of stable MTs that are enriched
in detyrosinated tubulin. Most stable MTs, which often
appear short, convoluted and with their ends rarely
extending to the cell periphery, concentrate around the
centrosome. The morphology of the GA closely follows
that of these stable MTs [1] and vice versa Ð some of
these MTs apparently having both ends anchored to the
GA (our observations). Thus, whereas dynamic MTs has
been involved in the early stages of Golgi membrane
minus-end-directed transport [14], stable MTs might be
important in the maintenance of GA structure and localisation. Stabilisation of MTs may be achieved by association of MT-associated proteins or by capping MT plus
ends [15]. Although a direct role has not been documented, the recently described CLIP-associated proteins
CLASPs [16] are good candidates to stabilise the subset
of MTs implicated in the organisation of the GA.
CLASPs, when overexpressed, exhibit MT-stabilising
effects and increase the number of detyrosinated MTs.
There is a substantial Golgi-associated pool of CLASPs,
and CLASP2 is targeted to Golgi membranes by fatty
acylation of two cysteine residues in its ®rst 14 amino
acids.
The Golgi apparatus and minus-endanchoring activities
In the textbook view, the centrosome appears as the
unique site for both MT nucleation (i.e. formation of a
new MT) and organisation, because newly formed MTs
are proposed to remain tightly bound to the pericentriolar
material. In this view, the GA is depicted as a ribbon
positioned near the MT aster. It has become clear, however, that centrosome activity is more complex and
dynamic. Most cells constantly release MTs from centrosomes that are then anchored to centrosomal and noncentrosomal sites. Non-centrosomal MT assembly and
organisation can also occur [17,18]. The mechanisms for
MT nucleation and anchoring at non-centrosomal sites
remains poorly understood. The extent of MT release
from the centrosome depends on the somatic cell type
and correlates with the concentration of MT-anchoring
activities at the centrosome [18]. Although with some
variations, the morphology of the GA in non-polarised
cells seems also to correlate with the same factors. At one
end of the spectrum, lymphoid cells, in which all of MTs
are anchored to the centrosome, display a spherical GA
surrounding the centrosome. Fibroblasts with wellCurrent Opinion in Cell Biology 2003, 15:60±66
focused MT asters, despite a certain level of MT-release
activity [17], have a pericentrosomal GA that is extended
into a juxtanuclear organisation. Finally, non-polarised
epithelial cells frequently display GA surrounding the
nuclear envelope. In such cells, many MTs do not seem
to be anchored to the centrosome but to the nuclear envelope.
Such an intimate relationship between GA and MTanchoring activities is also revealed during treatment of
cells with taxol or during cell differentiation. In lymphoblasts treated with taxol, stable MTs remain anchored to
the centrosome and Golgi localisation does not change
[18,19]. By contrast, in ®broblastic and epithelial cells,
taxol treatment induces stable MTs to form bundles away
from the centrosome. Most of the bundles recruit anchoring proteins and other centrosomal proteins to one end
[18]. Under these conditions, GA elements are also associated with this end of MT bundles (our observations).
During myogenesis, both the GA and the centrosome are
dramatically reorganised. The compact juxtanuclear GA
appears to disperse into elements that form a belt around
each of the myotube nuclei and extend between the
nuclei. This redistribution is accompanied by a similar
reorganisation of MTs, MT-nucleating sites and centrosomal proteins such as pericentrin [20,21]. It has now
been shown in living cells that after reorganisation, both
ER exit sites and Golgi elements are found near the MTnucleating centres and thus near the MT-minus ends
[22].
c-Tubulin ring complexes
The mechanisms involved in maintaining an association
between the GA and MT minus ends are unclear. An
attractive possibility is that MT-nucleating/anchoring
activities could be associated with the GA. These activities could serve to determine the central location of GA
within the cell and to contribute to the formation of a
specialised subcellular domain. At present, the best-characterised MT minus end anchors are the g-tubulin ring
complexes (g-TuRCs) that, in addition to their role in MT
nucleation, have been reported to act as MT minus end
caps [23]. A fraction of cytosolic g-tubulin has been found
recently to associate with puri®ed Golgi membranes [24].
The Golgi membrane fraction was reported to nucleate
MTs both in a permeabilised cell system and in in vitro
assays. This activity required peripherally associated
Golgi proteins, speci®cally g-tubulin. A co-localisation
of nocodazole (NZ)-induced Golgi elements and short,
stable MTs that appeared in the cell periphery after NZwashout in hepatic cells has also been shown. Whether
this co-distribution represents MT nucleation by Golgi
elements or growth from short, stable fragments, and
whether g-tubulin is involved in this process in vivo
remain to be determined. A good candidate for this
MT-nucleating or stabilising activity of the GA is
www.current-opinion.com
The Golgi apparatus at the cell centre Rios and Bornens 63
GMAP-210, which is able to recruit g-tubulin-containing
complexes to Golgi membranes (RM Rios and M Bornens, unpublished data).
Dynactin
Another protein complex proposed to play a role in MT
minus-end-anchoring at the centrosome is dynactin [25].
This large multisubunit complex is generally believed to
function as an adaptor or receptor for cytoplasmic dynein
on cargo, and it has been shown to be required for dyneinbased motility in vitro and in vivo. Dynactin consists of
two structural domains: an actin-like backbone, thought
to be responsible for cargo attachment; and a projecting
shoulder side arm, containing p150Glued, dynamitin and
p24 subunits, that interacts with cytoplasmic dynein as
well as with MTs [4,25]. In the interphase centrosome,
dynactin seems to act independently of dynein, and the
MT-binding activity of its p150Glued subunit is required
for the maintenance of a centrosomally focused aster. MT
nucleation by dynein±dynactin complexes is also
involved in the organisation of a radial microtubule array
in the absence of centrosomes in melanophore fragments
[26]. At the GA, dynactin is thought to promote dynein
docking to cargo, and available data support the model
that every effect of dynactin in the GA involves dynein
motor activity. However, perturbation of dynactin structure blocks the earliest events of Golgi biogenesis, thus
precluding evaluation of a putative static role of dynactin
at the GA. Live-cell imaging of transfected cells has now
revealed that p150Glued is targeted to the plus ends of
growing MTs, where it might play a novel role in the early
stages of minus-end-directed membrane transport [27].
A recently characterised dynamitin-interacting protein
Bicaudal D2 (BICD2) has also been found to co-localise
with dynactin at both the GA and MT plus ends [28].
It must be noted, however, that dynactin localises predominantly where minus ends concentrate and that
p150Glued is able to bind MTs along their lengths and
to induce MT-bundling at medium and high expression
levels. In addition, it has been reported that proteins such
as XMAP215, ®rst identi®ed in Xenopus egg extracts by its
ability to stimulate the growth rate of MTs at the plus
ends, and its orthologues might act on both minus and
plus ends [29]. Whether dynactin plays a role in anchoring
or stabilising MTs at Golgi membrane surfaces awaits
further investigation.
AKAP450
Several lines of evidence indicate that protein kinase A
(PKA) type II might be important for stabilising the
minus end of MTs that originate from the centrosome
[30]. PKA II is targeted to the Golgi/centrosome area by
interaction of its regulatory subunit with pericentrin [31]
and AKAP450 (also known as CG-NAP or AKAP350, [32±
34]). AKAP450 is the product of a multiply spliced gene
that generates numerous isoforms of a large protein that
www.current-opinion.com
scaffold many enzymes, typically protein kinases and
protein phosphatases. AKAP450 is localised at both the
centrosome and the GA [32,33]. Its localisation to the
centrosome is independent of MTs, whereas that to the
GA is disrupted by MT depolymerisation or BFA treatment [32]. Recent data suggest that AKAP450, which is a
substrate of PKA, plays a role in MT dynamics [35,36].
The amino-terminal domain of AKAP450 has been found
to interact with GCP2/GCP3 components of g-TuRC and
could provide sites for MT nucleation or anchoring [35].
Interaction of AKAP450 with members of the centrosomal MT-interacting TACC (transforming acidic coiledcoil-containing) protein family also supports this idea
[36]. Interestingly, p150Glued binding to MTs is regulated
by PKA phosphorylation [27].
New functions for the pericentrosomal
location of the Golgi apparatus
Why Golgi membranes are anchored to the pericentriolar
region in mammalian cells is a question that remains
unanswered. This localisation has been considered optimal for radial transportation of transport intermediates
derived from the ER to a central location along the MT
aster. However, MT-based motility is not absolutely
required for protein traf®cking. MTs have been proposed
to facilitate long-range membrane traf®c, whereas shortrange membrane movement, such as Golgi recycling or
ER export, is thought to be a MT-independent process.
Analysis of the distribution of ER exit sites has revealed
that although present throughout the ER network, many
of them are positioned at the perinuclear region, close to
the centrosome [37]. This distribution is rapidly modi®ed
in the absence of MTs [38]. When ER exit is blocked by
microinjection or overexpression of dominant-negative
Sar1 mutants, Golgi enzymes redistribute to the ER,
whereas Golgi matrix proteins accumulate at structures
that have been proposed to be ER exit sites [39,40].
Under these conditions, these structures appeared clustered around the centrosome. Moreover, as mentioned
above, during myoblast/myotube differentiation, Golgi
elements, ER exit sites and MT nucleation sites are
reorganised and relocated together [22]. The pericentrosomal region thus appears as a subcellular domain in
which essential activities for the functioning of the cytoskeletal and secretory systems concentrate. It has been
proposed that the radial array of MTs anchored to the
centrosome and projecting to the cell periphery could
provide the cell with a mechanism able to regulate MT
cytoskeleton as a whole by regulating centrosomal activity. Accumulation of the ER/Golgi system around the
centrosome could also serve a similar role and could
facilitate the control of cellular processes under conditions where cellular architecture as a whole is undergoing
remodelling. This occurs during cell migration, polarisation or differentiation. Noteworthy, the extent of the
centrosome, and thus focusing of the MT aster, varies
extensively during cell locomotion [41].
Current Opinion in Cell Biology 2003, 15:60±66
64 Cell structure and dynamics
A growing body of evidence indicates that the Golgi/
centrosome region could also play important roles in
several physiological processes, such as intracellular signalling, mitosis or apoptosis. The notion that both the
centrosome and the GA could act as signalling platforms
has been fostered by the identi®cation of a variety of
signal transduction molecules in these organelles [42±44].
AKAP450 [32±34] and myomegalin [45], both localised in
the GA and centrosome, have the capacity to bind several
kinases, phosphatases and phosphodiesterases, enzymes
involved in cAMP- and Rho-dependent signalling. Members of the Rho/Rac/Cdc42 family also accumulate in this
region and can apparently in¯uence not only speci®c
functions of GA or centrosome but also the coordinated
behaviour of both organelles.
The recently identi®ed ARAP (Arf-GTPase activating
[GAP], Rho-GAP, ankyrin repeat and pleckstrin homology domains-containing) proteins have been shown to be
components of the signalling pathway regulating cell
movement [46]. ARAP1 localises at the GA and regulates
Arf-, Rho- and Cdc42-dependent cell activities. It has
been proposed that ARAP1 coordinates membrane and
actin remodelling involved in cell movement [46]. Cdc42
also regulates the reorientation of the Golgi/centrosome
area in migrating ®broblastic cells [47,48], and, when
activated, recruits the effector kinase PAK4 to Golgi
membranes. PAK4 also participates in the regulation of
cell morphology and motility [49]. Interestingly, PAK4
activity seems to be involved in oncogenic transformation
[49], whereas Cdc42-mediated cell functions, including
induction of ®lopodia formation, cell spreading and GA
re-orientation, are inhibited by activation or overexpression of the tumour suppressor protein p53 [50].
Recent molecular evidence suggests that the centrosome
is involved in cell cycle checkpoint control and cell cycle
progression [18,44]. More surprisingly, the fragmentation
and dispersal of the GA has been found to be a prerequisite for entry into mitosis in mammalian cells [51].
It has been suggested that the pericentriolar GA organisation is a sensor for controlling entry into mitosis. Both
the GA and the centrosome may also initiate apoptosis by
speci®c stress sensors and relay apoptosis-modulating
signals to the rest of the cell [52,53].
Although far from being fully understood, all these data
point to the idea that the Golgi/centrosome might also
serve the cell as a central checking station for the status of
cytoplasmic organelles before any important decision on
the life cycle is undertaken.
Conclusions and future perspectives
The highly dynamic nature of the GA probably relies on a
wide array of molecular motors and MT-anchoring proteins. Regulation of these opposing but complementary
activities would allow the GA to maintain its steady-state
Current Opinion in Cell Biology 2003, 15:60±66
localisation while constantly receiving and generating
membranes.
Future studies should be directed towards the characterisation of the molecular machinery involved in regulating
the association of the GA with MTs in the pericentrosomal area throughout the cell division cycle. Which
mechanisms regulate the coordinated behaviour of both
GA and centrosome in cellular processes involving extensive MT reorganisation is also a major question. Finally,
how such a coordinated behaviour is harnessed to signalling pathways to lead to speci®c cellular responses will
also attract attention in the future.
Acknowledgements
We thank B Goud and AM Tassin for critical reading of the manuscript and
BJ Marsh for kindly providing us with ®gures. Work in the authors'
laboratories is funded by the Ministerio de Ciencia y TecnologõÂa, Spain, and
by the Centre Nationale de la Recherche Scienti®que and the Institut Curie,
France.
References and recommended reading
Papers of particular interest, published within the annual period of
review, have been highlighted as:
of special interest
of outstanding interest
1.
Thyberg J, Moskalewski S: Role of microtubules in the
organization of the Golgi complex. Exp Cell Res 1999,
246:263-279.
2.
Lippincott-Schwartz J: Cell cycle maintenance and biogenesis
of the Golgi complex. Histochem Cell Biol 2001, 116:93-103.
3.
Pelham HR: Traf®c through the GA. J Cell Biol 2001, 155:1099-1101.
4.
Allan VJ, Thompson HM, McNiven MA: Motoring around the
Golgi. Nat Cell Biol 2002, 4:E236-E242.
5.
Grissom PM, Vaisberg EA, McIntosh JR: Identi®cation of a novel
light intermediate chain (D2LIC) for mammalian cytoplasmic
dynein 2. Mol Biol Cell 2002, 13:817-829.
6.
Xu Y, Takeda S, Nakata T, Noda Y, Tanaka Y, Hirokawa N: Role of
KIFC3 motor protein in Golgi positioning and integration. J Cell
Biol 2002, 158:293-303.
This paper reports a role of KIFC3, a microtubule minus-end-directed
kinesin superfamily protein, in Golgi positioning at the cell centre. Authors
provide evidence that KIFC3 and cytoplasmic dynein work synergistically
to assemble the Golgi apparatus. Cholesterol is an important factor in this
process.
7.
Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin
LA: Golgi structure in three dimensions: functional insights
from the normal rat kidney cells. J Cell Biol 1999, 144:1135-1149.
8.
Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR:
Organellar relationships in the Golgi region of the pancreatic
beta cell line, HIT-T15, visualized by high-resolution electron
tomography. Proc Natl Acad Sci USA 2001, 98:2399-2406.
Forthe®rsttime,high-resolutionelectrontomography oftheGolgiregionhas
allowed visualisation of the three-dimensional associations of several subcompartments with the others and with microtubules after optimal preservation of cell structures. This study reveals that microtubules form close
associations with the cis-Golgi, the ER and the endo±lysosomal compartment. It also shows that the paths of individual microtubules closely follow
the membranes of the cis cisternae over considerable distances.
9.
Habermann A, Schroer TA, Grif®ths G, Burkhardt JA:
Immunolocalization of cytoplasmic dynein and dynactin
subunits in cultured macrophages: enrichment on early
endocytic organelles. J Cell Sci 2001, 114:229-240.
10. Infante C, Ramos-Morales F, Fedriani C, Bornens M, Rios RM:
GMAP-210, a cis-Golgi network-associated protein, is a minus
end microtubule-binding protein. J Cell Biol 1999, 145:83-98.
www.current-opinion.com
The Golgi apparatus at the cell centre Rios and Bornens 65
11. Pernet-Gallay K, Antony C, Johannes L, Bornens M, Goud B, Rios
RM: The overexpression of GMAP210 blocks anterograde and
retrograde transport between the ER and the GA. Traf®c 2002,
3:822-832.
12. Walenta JH, Didier AJ, Liu X, KraÈmer H: The Golgi-associated
Hook3 protein is a member of a novel family of
microtubule-binding proteins. J Cell Biol 2001, 152:923-934.
13. Perez F, Pernet-Gallay K, Nizak C, Goodson HV, Kreis TE, Goud
B: CLIPR-59, a new trans-Golgi/TGN cytoplasmic linker
protein belonging to the CLIP-170 family. J Cell Biol 2002,
156:31-642.
14. Vaughan KT, Tynan SH, Faulkner NE, Echeverri CJ, Vallee RB:
Colocalization of cytoplasmic dynein with dynactin and
CLIP-170 at microtubule distal ends. J Cell Sci 1999,
112:1437-1447.
15. Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG:
Detyrosinated (Glu) MTs are stabilized by an ATP-sensitive
plus-end cap. J Cell Sci 2000, 113:3907-3919.
16. Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland
B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld
F, Galjart N: CLASPs are CLIP-115 and -170 associating proteins
involved in the regional regulation of microtubule dynamics in
motile ®broblasts. Cell 2001, 104:923-935.
This paper reports the identi®cation of two CLIP-associated proteins
(CLASPs) that binds CLIPs and microtubules (MTs) and exhibit MT-stabilising properties. CLASPs localise at microtubule distal ends and seem to be
required for the orientation of stabilised MTs toward the leading edge of
migrating ®broblasts. CLASPs also accumulate in the Golgi region and
CLASP2 is directly targeted to Golgi membranes by fatty acylation.
17. Keating TJ, Borisy GG: Centrosomal and non-centrosomal
microtubules. Biol Cell 1999, 91:321-329.
18. Bornens M: Centrosome composition and microtubule
anchoring mechanisms. Curr Opin Cell Biol 2002, 14:25-34.
19. Knox JD, Mitchel RE, Brown DL: Effects of taxol and taxol/
hyperthermia treatments on the functional polarization of
cytotoxic T lymphocytes. Cell Motil Cytoskel 1993, 24:129-138.
20. Ralston E, Lu Z, Ploug T: The reorganization of the Golgi complex
and microtubules in skeletal muscle is ®ber type-dependent.
J Neurosci 1999, 19:10694-10705.
21. Ralston E, Ploug T, Kalhovde J, Lomo T: Golgi complex,
endoplasmic reticulum exit sites and microtubules in skeletal
muscle ®bers are organized by patterned activity. J Neurosci
2001, 21:875-883.
22. Lu Z, Joseph D, Bugnard E, Zaal KJM, Ralston E: Golgi complex
reorganization during muscle differentiation: visualization in
living cells and mechanism. Mol Biol Cell 2001, 12:795-808.
The reorganisation of the Golgi apparatus (GA) during differentiation and
fusion of myoblastic cells into mutinucleated myotubes is reinvestigated.
Using confocal time-lapse recordings of green ¯uorescent protein-bound
mannosidase in living myoblasts, the authors conclude that changes in
GA organisation result from combined changes in microtubule nucleation
and ER exit site localisation, which place the ER exit sites near microtubule minus ends.
23. Wiese C, Zheng YX: A new function for the gamma-tubulin ring
complex as a microtubule minus-end cap. Nat Cell Biol 2000,
2:358-364.
24. Chabin-Brion K, Marceiller J, Perez F, Settegrana C, Drechou A,
Durand G, Pous C: The Golgi complex is a microtubule-organizing
organelle. Mol Biol Cell 2001, 12:2047-2060.
25. Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer
TA: Dynactin is required for microtubule anchoring at
centrosomes. J Cell Biol 1999, 147:321-334.
26. Vorobjev I, Malikov V, Rodionov V: Self-organization of a radial
microtubule-array by dynein-dependent nucleation of
microtubules. Proc Natl Acad Sci USA 2001, 98:10160-10165.
The authors use real-time live-cell studies and microinjection of dynein
inhibitors to demonstrate that the organisation of a radial microtubule
array in cytoplasmic fragments of ®sh melanophore fragments lacking the
centrosome is achieved through dynein-dependent microtubule nucleation. They propose that this mechanism may cooperate with the centrosome in the organisation of a radial microtubule array.
www.current-opinion.com
27. Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT: A role
for regulated binding of p150Glued to microtubule plus ends in
organelle transport. J Cell Biol 2002, 158:305-319.
The authors extend previous work on the role of dynactin in organelle
transport by analysing p150Glued in living cells. This analysis revealed
targeting of GFP-p150Glued to the plus ends of elongating microtubules
where it appears to participate in the early stages of minus-end-directed
membrane transport. Binding of p150Glued to microtubules was shown to
be regulated by protein kinase A phosphorylation.
28. Hoogenraad CC, Akhmanova A, Howell SA, Dortland BR, De Zeeuw
CI, Willemsen R, Visser P, Grosveld F, Galjart N: Mammalian
Golgi-associated Bicaudal-D2 functions in the dynein-dynactin
pathway by interacting with these complexes. EMBO J 2001,
20:4041-4054.
This paper describes the characterisation of a novel Golgi-associated
dynamitin-interacting protein, Bicaudal D2. BICD2, which also co-localises with dynactin at microtubule plus ends, is proposed to play a role in
the dynein±dynactin interaction on the surface of membrane organelles.
29. Kinoshita K, Habermann B, Hyman AA: XMAP215: a key
component of the dynamic microtubule cytoskeleton. Trends
Cell Biol 2002, 12:267-273.
30. Carlson CR, Witczak O, Vossebein L, Labbe JC, Skalhegg BS,
Keryer G, Herberg FW, Collas P, Tasken K: CDK1-mediated
phosphorylation of the RIIalpha regulatory subunit of PKA
works as a molecular switch that promotes dissociation of
RIIalpha from centrosomes at mitosis. J Cell Sci 2001,
114:3243-3254.
31. Diviani D, Langeberg LK, Doxsey SJ, Scott JD: Pericentrin
anchors protein kinase A at the centrosome through a newly
identi®ed RII-binding domain. Curr Biol 2000, 10:417-420.
32. Witczak O, Skalhegg BS, Keryer G, Bornens M, Tasken K, Jahnsen
T, Orstavik S: Cloning and characterization of a cDNA encoding
an A-kinase anchoring protein located in the centrosome,
AKAP450. EMBO J 1999, 18:1858-1868.
33. Takahashi M, Shibata H, Shimakawa M, Miyamoto M, Mukai H, Ono
Y: Characterization of a novel giant scaffolding protein,
CG-NAP, that anchors multiple signaling enzymes to
centrosome and the GA. J Biol Chem 1999, 274:17267-17274.
34. Schmidt PH, Drans®eld DT, Claudio JO, Hawley RG, Trotter KW,
Milgram SL, Goldenring JR: AKAP350, a multiply spliced protein
kinase A-anchoring protein associated with centrosomes.
J Biol Chem 1999, 274:3055-3066.
35. Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y:
Centrosomal proteins CG-NAP and kendrin provide
microtubule nucleation sites by anchoring c-tubulin ring
complex. Mol Biol Cell 2002, 13:3235-3245.
36. Steadman BT, Schmidt PH, Shanks RA, Lapierre LA, Goldenring
JR: Transforming acidic coiled-coil-containing protein 4
interacts with centrosomal AKAP350 and the mitotic spindle
apparatus. J Biol Chem 2002, 277:30165-30176.
37. Bannykh SI, Rowe T, Balch WE: The organization of endoplasmic
reticulum export complexes. J Cell Biol 1996, 135:19-35.
38. Hammond AT, Glick BS: Dynamics of transitional endoplasmic
reticulum sites in vertebrate cells. Mol Biol Cell 2000,
11:3013-3030.
39. Miles S, McManus H, Forsten KE, Storrie B: Evidence that the
entire GA cycles in interphase HeLa cells: sensitivity of Golgi
matrix proteins to an ER exit block. J Cell Biol 2001, 155:543-555.
40. Ward TH, Polishchuk RS, Caplan S, Hirschberg K,
Lippincott-Schwartz J: Maintenance of Golgi structure and
function depends on the integrity of ER export. J Cell Biol 2001,
155:557-570.
41. Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M: The
respective contributions of the mother and daughter centrioles
to centrosome activity and behavior in vertebrate cells. J Cell
Biol 2000, 149:317-330.
42. Donaldson JG, Lippincott-Schwartz J: Sorting and signaling at
the Golgi complex. Cell 2000, 101:693-696.
43. De Matteis MA, Godi A, Corda D: Phosphoinositides and the
Golgi complex. Curr Opin Cell Biol 2002, 14:434-447.
Current Opinion in Cell Biology 2003, 15:60±66
66 Cell structure and dynamics
44. Lange BM: Integration of the centrosome in cell cycle control,
stress response and signal transduction pathways. Curr Opin
Cell Biol 2002, 14:35-43.
45. Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer
J, Jin SL, Conti M: Myomegalin is a novel protein of the Golgi/
centrosome that interacts with a cyclic nucleotide
phosphodiesterase. J Biol Chem 2001, 276:11189-11198.
46. Miura K, Jacques KM, Stauffer S, Kubosaki A, Zhu K, Hirsch DS,
Resau J, Zheng Y, Randazzo PA: ARAP1: a point of convergence
for Arf and Rho signaling. Mol Cell 2002, 9:109-119.
This paper describes the characterisation of a novel protein associated
with the Golgi apparatus that displays phosphatidylinositol-1,4,5-trisphosphate-dependent Arf-GTPase-activating protein (GAP) and Rho-GAP
activities. These activities regulate Arf-, Rho- and Cdc42-dependent cell
functions. ARAP1 is proposed to function as a node in signalling networks
that coordinate actin and membrane remodelling.
47. Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, P®ster
KK, Vallee RB, Gundersen GG: Cdc42, dynein, and dynactin
regulate MTOC reorientation independent of Rho-regulated
microtubule stabilization. Curr Biol 2001, 11:1536-1541.
48. Nobes CD, Hall A: Rho GTPases control polarity, protrusion, and
adhesion during cell movement. J Cell Biol 1999, 144:1235-1244.
49. Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff
JR, Jallal B, Smeal T: Requirement for PAK4 in the
anchorage-independent growth of human cancer cell lines.
J Biol Chem 2002, 277:550-558.
Current Opinion in Cell Biology 2003, 15:60±66
50. Gadea G, Lapasset L, Gauthier-Rouviere C, Roux P: Regulation of
Cdc42-mediated morphological effects: a novel function for
p53. EMBO J 2002, 21:2373-2382.
51. Sutterlin C, Hsu P, Mallabiabarrena A, Malhotra V: Fragmentation
and dispersal of the pericentriolar Golgi complex is required
for entry into mitosis in mammalian cells. Cell 2002,
109:359-369.
This paper reports that inhibition of Golgi fragmentation and dispersal
prevented entry of cells into mitosis. Authors propose that the pericentriolar localisation of the Golgi apparatus could serve as a sensor in
regulating entry into mitosis.
52. Ferri KF, Kroemer G: Organelle-speci®c initiation of cell death
pathways. Nat Cell Biol 2001, 3:255-263.
53. Piekorz RP, Hoffmeyer A, Duntsch CD, McKay C, Nakajima H, Sexl
V, Snyder L, Rehg J, Ihle JN: The centrosomal protein TACC3 is
essential for hematopoietic stem cell function and genetically
interfaces with p53-regulated apoptosis. EMBO J 2002,
21:653-664.
By generating de®cient mice, authors show that de®ciency in the
centrosomal protein TACC3, which causes an embryonic lethality involving several cell lines, is associated with a high rate of apoptosis and
expression of the p53 target gene, p21Waf1/Cip1. Abnormalities caused in
several cell types by TACC3 de®ciency could be rescued by combining
TACC3 and p53 de®ciencies. This support that TACC3 is a critical
component of the centrosome and its absence triggers p53-mediated
apoptosis.
www.current-opinion.com