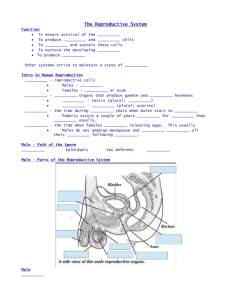
Gametes and Speciation-from prezygotic to postzygotic isolation
advertisement

Department of Animal Ecology Evolutionary Biology Centre Uppsala University Gametes and Speciation: from prezygotic to postzygotic isolation Murielle Ålund Introductory Research Essay No. 100 Uppsala 2012 ISSN 1404 - 4919 ISRN UU - ZEK - IRE -- 100 -- SE Gametes and Speciation: from prezygotic to postzygotic isolation Murielle Ålund Department of Animal Ecology Evolutionary Biology Centre Uppsala University Norbyvägen 18D SE-752 36 Uppsala Sweden ISSN 1404 - 4919 ISRN UU - ZEK – IRE -- 100 -- SE Contents Abstract ............................................................................................................................................... - 4 1. Speciation and the evolution of reproductive barriers ................................................................. - 5 - 2. Postmating prezygotic barriers or gametic isolation ................................................................... - 7 - 3. 4. 2.1 Sperm competition and conspecific sperm precedence ....................................................... - 9 - 2.2 Reproductive proteins and sperm-egg interactions ........................................................... - 11 - 2.3 Cryptic female choice and sexual conflict......................................................................... - 13 - Intrinsic postzygotic isolation: hybrid sterility .......................................................................... - 15 3.1 The Dobzhansky-Muller model......................................................................................... - 16 - 3.2 Haldane’s rule.................................................................................................................... - 18 - 3.3 Spermatogenesis and the genetics of hybrid male sterility................................................ - 19 - A model system for the study of pre- and postzygotic isolation: the Ficedula flycatchers....... - 21 - Conclusions ....................................................................................................................................... - 24 Acknowledgements ........................................................................................................................... - 25 References ......................................................................................................................................... - 25 - Abstract Speciation lies at the heart of evolutionary biology and researchers have been trying to understand the mechanisms leading to the evolution of reproductive isolation since over 250 years. Premating barriers (i.e. barriers preventing heterospecific individuals to mate with each other) and extrinsic postzygotic isolation (i.e. environmental factors affecting the fitness of hybrid individuals) have been studied in many taxa. However, little is known about what is happening at the gametic level, both before heterospecific fertilization (i.e. postmating prezygotic or gametic isolation) and in hybrid individuals (i.e. intrinsic postzygotic incompatibilities). In this essay, I will give an overview of the role gametes play in the evolution of reproductive isolation. I conclude that gametes and reproductive proteins evolve quickly, under strong influence of sexual and sexually antagonistic selection. Gametes are very diverse between species and sperm competition and female cryptic choice can lead to higher fertilization success of sperm from conspecific males. In the hybrid offspring, spermatogenesis can be easily disturbed by small differences in gene expression and this leads to a greater number of genes causing hybrid sterility compared to hybrid inviability among taxa. Following Haldane’s rule, the heterogametic sex is the first to be affected by hybrid incompatibilities, but different mechanisms seem to cause inviability and sterility and taxa with heterogametic males or heterogametic females might be affected differently. I end this review by focusing on one particular model system for studying speciation: the Ficedula flycatchers. Much is known about the ecological factors affecting speciation and hybridization between pied and collared flycatchers and new molecular data give insights into the genetics of speciation, but the role of gametes has not been studied in this system. Studies on gamete divergence and hybrid gamete production in the flycatchers will allow us to get a better idea of the role of gametes in speciation in a wild organism with homogametic males. -4- 1. Speciation and the evolution of reproductive barriers The existence, formation and maintenance of species as different entities have puzzled evolutionary biologists for over a century. A quick look at the number of publications including “The Origin of Species” in their title since Darwin’s book in 1859 gives an idea on the variety of fields working on the subject, including systematics (Mayr, 1942; Avise, 1997), genetics (Dobzhansky, 1937), molecular biology (Manwell & Baker, 1970; De la Cruz & Davies, 2000), ecology (Dieckmann & Doebeli, 1999; Schluter, 2001; Gavrilets, 2004) and behaviour (Van Doorn et al., 2009), among many others. The problem with studying how new species are formed is that speciation can rarely be observed directly since the process can take millions of years to be completed. As a result, researchers have to focus their studies on diverging populations or incipient species that might never become separate species, or work in the laboratory with species that diverged long ago (Rice et al., 2011). The definition of a species has been debated for decades and there is no uniformly accepted definition but at least 25 different “species concepts”. Scientists use different species concepts depending on the organism they are working with (sexual or asexual) and their questions of interest (systematics, genetics or ecology). The most widely used definition when describing sexually reproducing organisms is the Biological Species Concept, which states that individuals belonging to the same species can interbreed and produce viable and fertile offspring, while being reproductively isolated from individuals of other species (Mayr, 1942). Following this definition, research in speciation generally focuses on understanding the evolution of reproductive isolation (Coyne & Orr, 2004). Reproductive barriers, which include anything that impedes the exchange of genes between members of different populations, are classified in different categories, depending on when in the reproductive cycle they are acting. Prezygotic isolation mechanisms prevent individuals from choosing each other as mates (premating barriers) or impair fertilization success (postmating prezygotic barriers). Premating barriers can be caused by ecological isolation, when populations use different habitats or resources (habitat isolation) or when their breeding time is different (temporal isolation). Divergence in a secondary sexual character (e.g. colour, song or pheromones) can also reduce mate attractiveness between populations (behavioural isolation) or the morphology of the reproductive structures might simply be incompatible (mechanistic isolation). Finally, postmating prezygotic barriers or gametic isolation include all reproductive barriers acting between copulation and fertilization (see chapter 2). Postzygotic isolation mechanisms include different forms of selection against hybrids, either caused by extrinsic factors reducing their fitness (e.g. difficulties to find an ecological niche or to acquire a mate because of their intermediate phenotype) or intrinsic genetic incompatibilities leading to hybrid -5- sterility or inviability (Coyne & Orr, 2004, see chapter 3). Figure 1 below schematically summarizes the different forms of reproductive barriers between heterospecific individuals. Figure 1: Schematic representation of the reproductive barriers, starting with premating barriers preventing individuals from choosing each other as mates, then gametic isolation (postmating prezygotic) inside the female reproductive tract and finally picturing different forms of selection against hybrids (postzygotic barriers). Reproductive isolation is often thought to evolve as a by-product of ecological divergence in allopatry (Schluter, 2001). When there is a physical barrier to the exchange of migrants between populations, the populations will diverge over time, simply by genetic drift or by adaptation to different environments (resources, predators, competitors, etc.). Given enough time, this will lead to the evolution of reproductive isolation (Schluter, 2001). Premating barriers and behavioural isolation in particular can evolve relatively quickly and are reversible, as is extrinsic postzygotic isolation. Genetic incompatibilities on the other hand might take millions of years to appear. Mammals are generally still capable of producing hybrids after 2-3 millions, frogs after 21millions and birds after 22 million years (Coyne & Orr, 2004). Secondary contact, where populations are found in sympatry again after the disappearance of a physical barrier, is a crucial point in the speciation process. If there was too little divergence between the populations in allopatry, they might merge into one species again. Alternatively, premating barriers might be already so strong that there is no interbreeding at all: the species were formed in allopatry. Finally, hybridization might lead to the formation of unfit hybrids, in which case there should be natural selection for enhanced prezygotic barriers, a process called reinforcement (Grant et al., 1996; Schluter, 2001). The outcome of secondary contact will also be strongly influenced by environmental factors, including resources and territory availability, competition and predation (Schluter, 2001). In the more controversial scenario of sympatric speciation, reproductive isolation evolves without any geographical barrier while there is still free exchange of genes, due to a divergence in resources use coupled with strong assortative mating. The problem with this scenario is that hybridization and gene -6- flow between the diverging populations is likely to break down the associations between divergent traits and preference (Coyne & Orr, 2004). For sympatric speciation to occur, a linkage between the diverging trait and the associated preference, so-called “magic-trait” is needed, either through linkage disequilibrium at the molecular level or by having one trait simultaneously involved in ecological functions and in mate preference or species recognition (Servedio et al., 2011). It is generally accepted that speciation occurs as a combination of different reproductive barriers given enough divergence time, but it is difficult to know the relative importance of the different barriers and their order of appearance over time when observing complete species or species that just started diverging (Coyne & Orr, 2004; Rice et al., 2011). A solution to that may be to observe hybrid zones with species that came into secondary contact after a long period of divergence in allopatry, since it allows us to study the mechanisms evolving to avoid costly hybridization (Barton & Hewitt, 1985; Rice et al., 2011). In the next chapters, I will concentrate on two types of reproductive barriers involving gametes (i.e. sperm and egg): postmating prezygotic and intrinsic postzygotic isolation. In reviewing the existing literature, I will mostly concentrate on studies on animals, since it is the focus of my own work, although reproductive barriers have also been extensively studied in plants. 2. Postmating prezygotic barriers or gametic isolation Postmating prezygotic isolation, also called gametic isolation, includes any reproductive barrier acting between copulation and fertilization (Coyne & Orr, 2004; Howard et al., 2009). Postcopulatory prezygotic barriers are physiological and often difficult to investigate and as a result they are unrepresented among the studies on reproductive isolation (Coyne & Orr, 2004; Birkhead & Brillard, 2007; Martín-Coello et al., 2009). As discussed above, premating barriers and extrinsic postzygotic mechanisms evolve faster but are also easier to study, since they mostly depend on ecological factors. However, when premating isolation mechanisms are not efficient enough to prevent heterospecific individuals from mating (i.e. hybridization), postmating prezygotic reproductive barriers might play an important role in preventing the costly production of unfit hybrid offspring (Ludlow & Magurran, 2006; Immler et al., 2011). It is also likely to be more important in external than in internal fertilizers, particularly in broadcast spawners, since they are releasing gametes into the water without any premating interaction (Landry et al., 2003). Several factors can contribute to a reduced fertilization success in heterospecific mating compared to mating between individuals of the same species (i.e. conspecifics). First, there could be too few sperm transferred or a poor storage of the sperm in the female tract. A reduced gamete transfer and loss of sperm from the female tract were both observed in heterospecific mating in Drosophila (Price et al., -7- 2001; Sagga & Civetta, 2011). Furthermore, male gametes may have lower survival or an abnormal behaviour in a foreign reproductive tract, as was observed in the ground cricket where sperm was immotile in the reproductive tract of heterospecific females (Gregory & Howard, 1994). The proteins of the seminal fluid might also fail to stimulate ovulation or oviposition, as observed in Drosophila (Price et al., 2001). There may also be a lack of chemical attraction between gametes (e.g. in marine broadcast spawners, Miller, 1997) or incompatibilities between sperm and egg-recognition proteins (Vacquier, 1998, see section 2.3). Finally, when females mate both with conspecific and heterospecific males, conspecific sperm precedence is likely to occur (Servedio, 2001; Coyne & Orr, 2004, see section 2.1). Gametes and sperm in particular can evolve very quickly. Sperm morphology has been found to vary widely between closely related species in several taxa including mammals, insects and marine invertebrates (Howard et al., 2009). Even within populations, sperm morphology has been shown to vary between individuals in response to their social environment (Immler et al., 2010). A rapid change in morphology could contribute to differential fertilization success between species and thus to the rapid evolution of gametic isolation. Moreover, reproductive proteins are known to evolve quickly and several studies showed a rapid differentiation and strong selection pressures on genes coding for reproductive proteins in Drosophila and marine invertebrates (see section 2.3). Similarly, genitalia and female sperm storage organs also vary greatly across species (Howard et al., 2009). Gametic isolation has been observed throughout the animal kingdom, from corals and molluscs to mammals and birds. However, there is an overrepresentation of insects and marine invertebrates among studies on postmating prezygotic barriers. The later can be explained by the fact that broadcast spawners do not have any other form of premating barriers and there is thus strong selection on gamete compatibility. Insects on the other hand represent an extremely species-rich taxa and are very well suited for laboratory experiments, hence the abundance of data available for insect species (Howard et al., 2009). Gametic isolation involves many different barriers that can be more or less effective. If there is potentially strong pressure to avoid heterospecific mating in particular situations, it might also be costly to develop too strong barriers that would even impair conspecific mating. If gametic isolation has already been observed between populations at the intraspecific level (Coyne & Orr, 2004), it can also take millions of years to be totally effective, in birds for example (Birkhead & Brillard, 2007). In the following sections, I will in more detail describe two specific mechanisms of gametic isolation: conspecific sperm precedence and interaction between reproductive proteins. I will also discuss the role of female cryptic choice and sexual conflict in the evolution of reproductive isolation. -8- 2.1 Sperm competition and conspecific sperm precedence Sperm competition occurs whenever ejaculates from several males compete for the fertilization of the same set of ova (Parker, 1970). Polyandry is known to occur in most taxa, and as females copulate with several males, sperm of different males might co-occur in the female reproductive tract at a given time and compete for fertilization. There will be selection for traits that increase the fertilization success of sperm and females can exert sperm selection or female cryptic choice and bias the fertilization success in favour of certain males (Eberhard, 1996; Pizzari & Parker, 2009, see section 2.3). Sperm number and sperm quality are the two major determinants of an ejaculate’s fertilization success. Sperm size, morphology, swimming velocity, metabolic performance, longevity and seminal fluid peptides are all thought to contribute to sperm quality (Pizzari & Parker, 2009). The relationship between sperm size or morphology and the outcome of sperm competition is still not well understood (Pizzari & Parker, 2009). Over 20 years ago, Gomendio and Roldan proposed that longer sperm swim faster (Gomendio & Roldan, 1991) and this has been confirmed by numerous studies since then (reviewed in Helfenstein et al., 2009; Pizzari & Parker, 2009). The relationship between sperm size and longevity is still debated and contradicting results have been reported in different studies (Helfenstein et al., 2009). A trade-off between sperm velocity and longevity is often assumed, the idea being that fast sperm expend all their energy quickly and thus suffer earlier senescence (Pizzari & Parker, 2009). If long sperm swim faster and also expend their energy earlier, small sperm are likely to live longer and there should be a trade-off between the production of short, long-lived sperm and long, fast-swimming ones. When there is little sperm competition, it might be more beneficial to have longliving sperm that will remain long enough in the reproductive tract to fertilize a high number of eggs (Pizzari & Parker, 2009). If sperm competition is high on the other hand, there will not be any benefit of having long-lived sperm, as the number of competitors will increase over time and the priority will be to invest in highly competitive ejaculates containing sperm, which are able to reach the ovum first directly after insemination (before the female re-mated) (Engqvist, 2012). Different selection pressures or different strategies are likely to be found in different populations and this could have consequences for the outcome of sperm competition at secondary contact after heterospecific mating. Hybridization will be more likely to occur between a male from a population with high sperm competition and a female from a population with low sperm competition than the opposite, leading to asymmetric or unidirectional hybridization, as observed in three species of mouse for example (Birkhead & Brillard, 2007; Martín-Coello et al., 2009). “Conspecific sperm precedence” is defined as the capacity of sperm from conspecific males to gain more fertilizations than sperm from heterospecific males in situations of sperm competition (Howard, 1999). This can happen either because conspecific sperm outcompetes heterospecific sperm in the race to the ovum (at any stage from the entry in the female reproductive tract to the deposition in storage -9- organs to the actual fusion of the sperm with the ovum), or because heterospecific sperm fails to fertilize the egg. In both cases, the end result is the production of fewer hybrid offspring than would be expected given the proportion of heterospecific mating (Howard, 1999). Conspecific sperm precedence is potentially a very strong reproductive barrier when females randomly mate with conspecific and heterospecific males. However, as one species becomes rarer and the rate of encounter of a conspecific male decreases, more hybrid offspring will be produced and gene flow will increase if postzygotic barriers are not strong enough (Howard, 1999). Studies on conspecific sperm precedence are often needed to detect a barrier at the gametic level between species. Some studies failed to find any incompatibility between species until sperm precedence was analysed, as heterospecific sperm may be able to fertilize the egg in absence of competition with conspecific sperm (Howard et al., 2009). This is the case for the ground cricket Allonemobius fasciatus and its sister species A. socius, which produce fertile hybrid offspring when a female is mated to a heterospecific male only, while fertilization by heterospecific sperm rarely occurs when females are mated to both conspecific and heterospecific males (Howard et al., 1998). Conspecific sperm or pollen precedence seems to be a wide-spread mechanism reducing hybridization and has been identified in Drosophila (Price et al., 2000), mouse (Martín-Coello et al., 2009), flour beetles (Wade et al., 1994), grasshoppers (Hewitt et al., 1989), mussels , Iris, monkey flowers, sunflowers (reviewed in Howard, 1999 and Coyne & Orr, 2004) and even at the intraspecific level between populations of guppies separated for two million years (Ludlow & Magurran, 2006). Among external fertilizers, conspecific sperm precedence was observed in one type of heterospecific crossing but not in the other in two hybridizing sunfish species (Immler et al., 2011) and in sea urchins, only very few eggs are fertilized by heterospecific sperm compared to conspecific (Lillie, 1921). The latter is due to the specificity of sperm and egg binding proteins, which will be discussed in the following section. When females are in contact with both conspecific and heterospecific males, multiple mating and mechanisms of conspecific sperm precedence allow the female to minimize gamete waste in the production of unfit hybrid offspring or eggs that will not hatch. This was observed in two closely related species of ladybirds which show only 5% of egg hatching in heterospecific compared to conspecific mating, but achieve normal hatching rates when females were mated to both heterospecifics and conspecifics (Nakano, 1985). However, if mechanisms of conspecific sperm precedence are likely to evolve as by-products of differential sperm competition pressures in different populations and will be advantageous for females when premating barriers are incomplete, selection for postmating prezygotic barriers is unlikely in males since it contributes to the waste of gametes they already inseminated in the female (Coyne & Orr, 2004). The potential sexual conflict resulting from this will be discussed in section 2.3. - 10 - 2.2 Reproductive proteins and sperm-egg interactions Reproductive proteins are usually defined as proteins acting after mating by influencing gamete transfer, usage, storage and fertilization (Swanson & Vacquier, 2002b). Reproductive genes are among the 10% most rapidly evolving genes and a high divergence in reproductive proteins has been found in many different organisms, including marine ciliates, unicellular green algae, fungi, Arabidopsis, abalones, sea urchins, Drosophila, crickets, rodents and primates (Swanson & Vacquier, 2002a; b; Clark et al., 2006; Turner & Hoekstra, 2008; Marshall et al., 2011). The most well-known cases of rapid evolution of reproductive proteins are found in abalones and sea urchins, both marine invertebrates and broadcast spawners. These external fertilizers lack any form of behavioural interaction before mating, and gamete recognition proteins are thus likely to be an important barrier to heterospecific mating. In the abalone, the sperm contains lysin in its acrosomal capsule, which dissolves the vitellin envelope of the egg. Lysin has to bind to a receptor on the vitellin envelope named VERL in order to create a hole in which the sperm can swim (Turner & Hoekstra, 2008). This species-specific protein complex has been shown to evolve 50 times faster than the fastest evolving mammalian proteins (Swanson & Vacquier, 2002a). In sea urchins, bindin, a protein binding to a receptor on the egg surface is also evolving fast and is under positive selection (Turner & Hoekstra, 2008). In internal fertilizers, the sperm has to pass multiple barriers before reaching and fertilizing the ovum (see Figure 1). Numerous proteins play an important rule at different stages of this process (reviewed in Clark et al., 2006) When sperm enters the reproductive tract, it has to face different pathogens and resist against the female immune system. Antibacterial proteins and prostaglandin decreasing female immune response have been observed in Drosophila and mammals. Chemotaxis (chemical attraction) between the egg and the sperm can be found in humans, facilitating the navigation of sperm towards the egg. Just after ovulation, both sperm and egg have to go through maturation for fertilization to be possible, a mechanism that is likely to be controlled by specific substances released in the oviduct. Sperm is often stored in the female reproductive tract, from a few days to several months or even over years. Seminal proteins have been shown to affect sperm storage in Drosophila. When reaching the ovum, the sperm first has to open a hole in the egg coat and then bind to the egg membrane and fuse with the nucleus, a process that is controlled by species specific molecules and enzymes present in the sperm head and on the egg membrane (Clark et al., 2006). In mammals, one of these proteins is the zona pellucida glycoprotein 3 (ZP3), one of the egg-coat proteins, which is binding sperm and has been shown to be under positive selection (Turner & Hoekstra, 2008). Protein involved in all the steps mentioned above as well as seminal proteins inducing ovulation or controlling females remating rates and ion-channels influencing sperm motility have all been shown to - 11 - be rapidly diverging between closely related species in different taxa (Howard, 1999; Swanson & Vacquier, 2002a; b; Clark et al., 2006; Turner & Hoekstra, 2008). In Drosophila, over 100 accessory gland proteins (Acps) have been identified in the seminal fluid and affect various processes in the female reproductive tract and they are twice as diverse as non-reproductive proteins (Swanson & Vacquier, 2002a; Turner & Hoekstra, 2008). Recently, proteomic analyses have allowed the study of protein contents of ejaculates of non-model species as well. This was the case in crickets for example, where a comparison between the proteomes of two closely related species showed high divergence and positive selection on ejaculates, indicating that the rapid evolution of reproductive isolation through postmating, prezygotic isolation is possible in 30’000 years only (Marshall et al., 2011). The use of molecular methods also allows broader comparisons of selective forces acting on gamete recognition genes. Berlin and colleagues showed evidence of positive selection on six gamete-recognition genes across several species of birds and concluded that the selection mechanisms were similar to those found in mammals (Berlin et al., 2008). Different hypotheses have been proposed to explain the fast evolution of reproductive proteins (Howard, 1999; Swanson & Vacquier, 2002b; Findlay & Swanson, 2010). One common explanation for fast divergence of reproductive proteins is pathogen avoidance. Both eggs of marine invertebrates and the reproductive tract of internal fertilizers are exposed to all kinds of pathogens and female egg coat proteins are thought to diverge rapidly in order to counter-act rapid pathogen evolution (Howard, 1999; Swanson & Vacquier, 2002b; Findlay & Swanson, 2010). Another hypothesis is reinforcement or selection against hybridization, where reproductive proteins evolve faster in sympatry than in allopatry to prevent costly heterospecific fertilization. This will most likely act on egg proteins to avoid fertilization by the wrong sperm, since egg production is costlier than the production of numerous sperm. Furthermore, once sperm has been released, any mechanism reducing the chance of fertilization will induce a waste of this sperm. Reinforcement of gamete isolation has been observed in sea urchins (Palumbi, 2009) and in Drosophila (Matute, 2010). In the latter example, a lower proportion of fertilized eggs following heterospecific mating were observed in a hybrid zone compared to similar crosses between allopatric populations. Furthermore, heterospecific sperm loss was more pronounced between sympatric than between allopatric populations (Matute, 2010). Interestingly, populations experimentally kept in sympatry showed signs of enhanced gametic isolation after four generations only (Matute, 2010). Finally, sexual selection on gametes (or cryptic female choice) and sexual conflict have been hypothesized to drive the fast evolution of reproductive proteins (Howard, 1999; Swanson & Vacquier, 2002b; Findlay & Swanson, 2010). These two mechanisms are described in more details in the following section. - 12 - 2.3 Cryptic female choice and sexual conflict Cryptic female choice is defined as any mechanism acting after copulation that biases paternity towards one male (Eberhard, 1996). The female reproductive tract consists of a variety of strong barriers that allow only sperm of vigorous or so-called “good quality” males to fertilize the egg (Pitnick et al., 2009). Through polyandry and extra-pair copulations, situations will occur where sperm of different males compete for the fertilization of the same egg and this should allow female to “select” the sperm of certain males over others. There are three major competing hypotheses describing the benefit of such choice. According to the sexually selected sperm hypothesis, fertilization by males with sperm of superior competitive ability will result in sons with highly competitive sperm and hence higher reproductive success. The good sperm hypothesis on the other hand proposes that males with good sperm are also of higher overall genetic quality (Pitnick et al., 2009). Finally, the genetic compatibility hypothesis suggests that cryptic female choice functions as a mechanism to avoid fertilization by sperm of genetically incompatible males, possibly through antisperm immune reactions (Zeh & Zeh, 1997). A positive relationship between sperm competitive ability and offspring fitness was found in the yellow dung fly and in a marsupial, while links between condition and sperm characteristics were observed in red deer, guppies, Atlantic cod, dung beetle and Drosophila (Pitnick et al., 2009). A recent study showed biased fertilization towards sperm carrying particular MHC alleles in a socially monogamous bird (Alcaide et al., 2012), a mechanism that could both be linked to genetic compatibility or superior genetic quality, but no data on offspring fitness were analysed. Fertilization bias against inbreeding has been more extensively studied and was reported in mice, lizard, field cricket, Drosophila and C. elegans (Pitnick et al., 2009). The exact mechanisms involved in female cryptic choice are difficult to study and still poorly understood. Females can bias fertilization towards a particular genotype either by using physical or chemical barriers or by actively ejecting sperm (Zeh & Zeh, 1997; Pitnick et al., 2009). In birds for example, less than 2% of the sperm ever reaches the storage organs (Pitnick et al., 2009). These strong barriers select for offensive adaptations in males, evolving aggressive ejaculates with higher quantity or faster sperm, more efficient at finding fertilization sites or at depletion of previous sperm, among other characteristics (Arnqvist & Rowe, 2005). However, a highly fertile ejaculate will in turn enhance the risk of polyspermy (the penetration of the egg by several sperm at the same time). Polyspermy is often detrimental to the embryo (it is lethal in mammals for example), thus it is in the interest of the female to evolve resistance against it (Palumbi, 2009). This situation of conflict between the evolutionary interests of the two sexes (here males willing to enhance their fertilization success and females avoiding polyspermy and selecting for sperm quality) is more generally termed sexual conflict (Parker, 1979). It will usually result in sexually antagonistic coevolution or coevolutionary arm race between the sexes with the evolution of persistence adaptations in males and enhanced resistance in - 13 - females (Arnqvist & Rowe, 2005, see Figure 2). This can lead to extreme cases of indirect costs to females with for example the evolution of harmful genitalia or injection of sperm directly into the haemolymph in insects. On the other hand, the evolution of too strong barriers by the females might result in no fertilization at all (Arnqvist & Rowe, 2005). Figure 2: Schematic representation of sexually antagonistic selection at the gametic level or the co-evolutionary arm race occurring between gametes of the two sexes in polyandrous organisms. Sexual conflict and sexually antagonistic evolution are thought to drive the evolution of a variety of reproductive proteins in both sexes. The best described system here again is Drosophila, where male accessory gland proteins have been found to increase egg production, laying and ovulation, influence sperm competition, storage and utilization, enhance courtship receptivity, decrease food intake and suppress immune response of females. Some proteins also induce changes in the conformation of the female reproductive tract (e.g. induced muscle contraction to push sperm towards the storage organs). These accessory proteins have been shown to decrease longevity in female Drosophila (Arnqvist & Rowe, 2005). Female proteins can in turn induce changes in the ejaculate, including sperm maturation and activation of certain proteins (Arnqvist & Rowe, 2005; Pitnick et al., 2009). This continuous arm race between males and females leads to a very fast evolution of reproductive traits and the maintenance of a high diversity despite their evolutionary importance. This in turn will eventually lead to reproductive isolation between allopatric populations (Arnqvist et al., 2000). In a comparative study, Arnqvist and colleagues showed a four-fold increase in speciation rates in polyandrous insect clades, where there is more opportunity for sexual selection and sexual conflict, - 14 - compared to monoandrous clades where sperm competition and female cryptic choice is unlikely to occur (Arnqvist et al., 2000). This demonstrates the importance of polyandry, sexual selection and sexual conflict in the evolution of reproductive isolation and hence the formation of new species. 3. Intrinsic postzygotic isolation: hybrid sterility In the previous section of this essay, I explored several mechanisms preventing mating between individuals of different species. Prezygotic reproductive barriers are numerous and diverse, however, also often incomplete. When individuals of different populations lack complete reproductive isolation at secondary contact or in sympatry, this can lead to hybridization. Hybridization is quite common in nature, according to a survey by Mallet, it occurs in 10% of all animal species and 25% of all plant species at least (Mallet, 2005). Similarly, 10% of all bird species hybridize or have hybridized (Grant & Grant, 1992). In general, hybrids perform poorly as compared to their parents (Burke & Arnold, 2001) and different form of selection against hybrid individuals are grouped under the term “postzygotic isolation”. Extrinsic postzygotic isolation refers to lower hybrid fitness due to a poor match with its environment (e.g. difficulties to acquire resources or mates due to intermediate phenotype), while intrinsic postzygotic isolation generally describes hybrid sterility and/or inviability due to genetic incompatibilities (Turelli et al., 2001; Coyne & Orr, 2004). Intrinsic postzygotic isolation can be caused by different ploidy levels, different chromosomal rearrangements (chromosomal speciation), divergent alleles not functioning together (DobzhanskyMuller incompatibilities, see section 3.1) or an infection by different endosymbionts causing cytoplasmic incompatibilities (Coyne & Orr, 2004). Chromosomal speciation is more common in plants than animals since self-fertilization increases the probability of fixation of underdominant rearrangements and fertility can be restored in plants through higher ploidy levels (e.g. tetrapoloidy for diploid plants). In animals, the presence of degenerated sex chromosomes (Y or W) enhances the probability of the evolution of genetic incompatibilities (Coyne & Orr, 2004). Cytoplasmic incompatibilities can be caused by different infectious agents, of which Wolbachia is a well-studied example. This bacterial parasite is maternally transmitted and induces the disruption of paternal chromosome processing /and or mitosis, causing hybrid inviability when populations adapted to different strains of Wolbachia meet at secondary contact (Coyne & Orr, 2004). Postzygotic isolating mechanisms are generally less studied than premating barriers and most of the literature on postmating isolation is biased towards Drosophila and other invertebrates (Birkhead and Brillard, 2007). The first studies of hybrid sterility were performed at the beginning of the last century, - 15 - generally in domestic animals. These experiments consisted of crossing different species of equids and bovids (Crew & Koller, 1936; Craft, 1938), ducks (Rigdon & Mott, 1965) and phasianids (Yamashina, 1943) and performing cytological analyses on the hybrid offspring. The outcome of numerous interspecific crosses have been described since then, mostly in captive (Johnsgard, 1970; Price & Bouvier, 2002; Lijtmaer et al., 2003) and wild derived populations crossed in the laboratory (e.g. Close et al., 1992; Kopp & Frank, 2005; Britton-davidian et al., 2005; Borodin et al., 2006; Good et al., 2008). More recently, studies have focused on the genetics of intrinsic postzygotic incompatibilities, in the search of “speciation genes”, mainly in mice and Drosophila, typically crossing laboratory species that diverged long ago (reviewed in Turelli et al., 2001; Orr, 2005; Presgraves, 2010). A general pattern emerging from these studies is that genes causing hybrid sterility seem to be affecting one sex only and they are ordinary genes having normal functions within species (Coyne & Orr, 2004). A major problem when studying species that are completely reproductively isolated is that it is often impossible to determine the origin of hybrid sterility or inviability, because many genetic incompatibilities will have accumulated over time (Orr, 1995; Turelli et al., 2001; Rice et al., 2011). A solution to this problem is to study incipient speciation or hybrid zones where species started diverging in allopatry and have come into secondary contact before being completely reproductively isolated (Barton & Hewitt, 1981; Grant & Grant, 1992; Rice et al., 2011). Hybrid sterility has been studied in some wild-derived populations of house mice and Drosophila (Kopp & Frank, 2005; Britton-davidian et al., 2005; Good et al., 2008) but it remains difficult to detect in nature, since hybrids need to be identified and their fitness has to be quantified accurately (The Marie Curie Speciation Network, 2012). As a result, very few studies describe intrinsic genetic incompatibilities in hybrids resulting from natural hybridization (see Rhymer & Simberloff, 1996) and these studies are usually based on single or scarce observations of hybrid individuals (e.g. Reifová et al., 2011). 3.1 The Dobzhansky-Muller model When hybridization occurs at secondary contact between populations that have diverged in allopatry (i.e. geographically isolated), some alleles that have never been in contact before might interact in the hybrid genome, provoking genetic incompatibilities and possible dysfunctions, since there was no coevolution between those alleles for many years (Moehring, 2011). This is known as “DobzhanskyMüller incompatibilities” (Dobzhansky, 1936; Müller, 1940). Figure 3 below describes this phenomenon in more details. - 16 - Figure 3: Dobzhansky-Muller model: different mutations occur in different populations in allopatry and the new alleles, which have never co-occurred in the same genetic background before, cause incompatibilities in the hybrids. When populations are isolated from each other for a long time, even if they experience similar environmental conditions, they will accumulate divergent mutations just by chance. This is because genomes are so complex that there are many different routes to the same adaptation (Turelli et al., 2001). New alleles emerging in each separate population will be well adapted to their own background genome, but may cause incompatibilities when they occur together with genes that they have not coevolved with in hybrid individuals. Through this process, alleles that have positive effects in one population can be detrimental in a hybrid background, and hybrid incompatibilities can arise without any fixation of “negative”-effect allele and without maladaptive intermediates (Turelli et al., 2001). The model above describes a very simple case where only one allele diverges in each population. Dobzhansky-Muller incompatibilities typically follow a “snowball effect”: genetic divergence increases linearly with diverging time and incompatibilities contributing to postzygotic isolation increase at least to the square of the divergence time (Turelli et al., 2001). This is because each new mutation increases the number of possible combinations resulting in incompatibilities (Coyne & Orr, 2004). The Dobzhansky-Muller model for the evolution of hybrid incompatibilities describes incompatibilities between diverging regions of the nuclear genome, but it is also possible that interactions between cytoplasmic (from the chloroplast or the mitochondria) and nuclear genes produce similar hybrid incompatibilities (Burke & Arnold, 2001). Hybrid sterility and inviability evolving as by-products of divergence of genomes in allopatry have been confirmed mostly in Drosophila and in plants (reviewed in Coyne & Orr, 2004), but also in hybrids between the platyfish and the swordtail (Wittbrodt et al., 1989) and between Nasonia wasp species (Gadau et al., 1999). - 17 - 3.2 Haldane’s rule Haldane’s rule describes a pattern observed among hybrid offspring of many taxa regarding hybrid incompatibilities: “When in the F1 offspring of two different animal races one sex is absent, rare, or sterile, that sex is the heterozygous sex” (Haldane, 1922). What Haldane described as “heterozygous” is now commonly termed “the heterogametic sex”, i.e. the sex having one single copy of each sex chromosome. In mammals and many insects, males are heterogametic (XY) and females are homogametic (XX), while in birds and Lepidoptera, the opposite pattern is the rule (heterogametic females (WZ) and homogametic males (ZZ)). Haldane’s rule is obeyed is all taxa surveyed so far, for both inviability and sterility (Wu & Davis, 1993). Independently of which sex is the heterogametic one, it is the first one to be affected by incompatibilities (Coyne & Orr, 2004). In birds, female sterility evolves first, but male sterility generally appears before female inviability (Price & Bouvier, 2002), as opposed to Drosophila where the heterogametic sex (males) loses both fertility and viability long before the homogametic sex (females) is affected (Coyne & Orr, 1997). In Lepidoptera, Haldane’s rule is obeyed equally for sterility and inviability (the female is affected first in both cases) (Presgraves, 2002). Hybrid sterility evolves faster than inviability and in 38% of the crosses observed by Presgraves, complete female inviability evolves before male sterility in hybrids. One of the most widely accepted explanations for Haldane’s rule is the dominance theory (Muller & Pontecarvo, 1942). If genetic incompatibilities exist between sex chromosomes and autosomes, the heterogametic sex will suffer from recessive and dominant incompatibilities, while the homogametic sex will only be affected by dominant incompatibilities, having a second copy of the sex chromosome to compensate for recessive incompatibilities (Muller & Pontecarvo, 1942). Another possible cause for the observed phenomenon (in species with heterogametic males) is the faster X theory, based on the idea that genes on the X chromosome evolve faster than autosomes, as they are exposed to selection in the heterogametic sex (Charlesworth et al., 1987). Moreover, the faster male theory argues that stronger sexual selection on males might cause faster evolution of male expressed genes, and that spermatogenesis might be more sensible than female gamete production (Wu & Davis, 1993, see next section for more details on spermatogenesis and hybrid male sterility). The faster X and the faster male theories only seem to be valid when describing hybrid sterility, and not hybrid inviability, and do not explain Haldane’s rule in taxa where the female is the heterogametic sex (Coyne & Orr, 2004). Finally, meiotic drive, or the distortion of segregation away from Mendelian ratios, has been proposed to explain hybrid male sterility (Frank, 1991). Alleles containing a drive will be transmitted to more than 50% of the offspring and will distort sex ratio if they are found on a sex chromosome (elimination of all sperm carrying a Y chromosome if the drive is found on the X chromosome for example). This will lead to a rapid coevolution of drivers and suppressors, increasing the rate of divergence between species. Hybrid offspring might end up with a driver but lose the corresponding suppressor through - 18 - recombination (McDermott & Noor, 2010). This would lead to hybrid male sterility if a male carries different drivers present on different sex chromosomes, which would lead to the destruction of both sperm carrying X and Y chromosomes (Coyne & Orr, 2004). All in all, none of the theories mentioned above seem to explain both the observed rates of hybrid inviability versus sterility among crosses and the evolution of hybrid incompatibilities in both taxa with heterogametic males and taxa with heterogametic females (Wu & Davis, 1993). It therefore seems likely that Hadane’s rule is a composite phenomenon, with several different mechanisms affecting the evolution of hybrid inviability and hybrid sterility (Wu & Davis, 1993). Hybrid inviability is probably easier to explain because lethal mutations are likely to affect both sexes in the same way, and Muller’s dominance theory (i.e. recessive interactions between autosomes and hemizygous sex chromosomes) seem to be enough to explain Haldane’s rule for inviability among all taxa (Wu & Davis, 1993). The faster-male hypothesis coupled to the dominance theory could explain the higher occurrence of hybrid sterility versus hybrid inviability, particularly in taxa with heterogametic males (Coyne & Orr, 2004). Different sets of genes affect male and female sterility and incompatibilities are likely to accumulate at different rates in the different sexes (Turelli et al., 2001). This could explain why the homogametic sex suffers from sterility before the evolution of inviability of the heterogametic sex in birds for example, or the observation of male sterility first in species where males have two full sets of genes on their sex chromosomes (e.g. Aedes mosquitoes having a fully functional Y chromosome, Presgraves & Orr, 1998) or where both sexes are functionally hemizygous (e.g. marsupials, Watson & Demuth, 2012). In the following section, I will explore the fast evolution of hybrid male sterility in more details. 3.3 Spermatogenesis and the genetics of hybrid male sterility Spermatogenesis seems to be one of the first mechanisms to be affected by hybrid incompatibilities in species where the heterogametic sex is the male (Haldane, 1922). Low sperm numbers have been observed in hybrid males of the parasitic wasp Nasonia (Clark et al., 2010) and between two species of Lepomis sunfish (Immler et al., 2011), whereas a loss of sperm motility and other alteration of sperm functions were described in hybrids Drosophila mojavensis x D. arizonae (Reed & Markow, 2004). A failure of hybrid males to produce mature sperm has been repeatedly observed in Drosophila (Wu & Davis, 1993; Clancy et al., 2011), sticklebacks (Takahashi et al., 2005), mice (Britton-davidian et al., 2005; Jeffrey M Good et al., 2008; Oka et al., 2010) and other rodents (Borodin et al., 2006). The mechanisms leading to the failure of producing mature and functional sperm were already analysed in detail by Dobzhansky in hybrids between two “races” (as he called them) of Drosophila pseudoobscura (Dobzhansky, 1934). In a comparison of the spermatogenesis of pure and hybrid - 19 - individuals, Dobzhansky described abnormalities in the first meiotic division and the absence of the second meiotic division, resulting in the production of “large worm-like spermatids”. In birds, where males are the homogametic sex, the absence of meiotic division and the consequent lack of gamete production was found to be the cause of sterility in hybrids between the domestic fowl and the common pheasant (Yamashina, 1943) and in hybrid ducks (Crew & Koller, 1936; Rigdon & Mott, 1965), where the presence of abnormal spermatids was also observed. The general pattern seems to be that spermatogenesis stops at early stages of the process (typically before or during the first meiosis) in sterile males, leading to the failure of producing mature sperm (Dobzhansky, 1934; Yamashina, 1943; Britton-davidian et al., 2005; Borodin et al., 2006; Good et al., 2008; Oka et al., 2010; Clancy et al., 2011). Some studies have found multiple genes on the X chromosome to be at the origin of spermatogenesis disruption in Drosophila (e.g. Tao et al., 2003) and mice (e.g. Dzur-Gejdosova et al., 2012), while others report an effect of mitochondrial elements, the cytochrome C in particular (Clancy et al., 2011). A recent study on Drosophila identified segregation distorters on the X-chromosome to be the origin of hybrid sterility (only sperm carrying Xchromosomes are viable in those hybrids that are partially fertile) (Phadnis & Orr, 2009). Several authors also mention an asymmetry in the sterility of hybrid males depending on the direction of the cross at early stages of speciation (Reed & Markow, 2004; Kopp & Frank, 2005; Britton-davidian et al., 2005; Good et al., 2008; Moehring, 2011), suggesting that incompatibilities might depend on the origin of the X-chromosome or the maternally inherited element and can be caused by a relatively small number of loci. Spermatogenesis seems to be particularly sensitive to any perturbation in gene expression. Sun and colleagues demonstrated that genes that only have minor influence on the fertility of pure-species Drosophila males can have detrimental consequences for hybrid fertility (Sun et al., 2004). Similarly, in a comparison between pure species and hybrid Drosophila males, Michalak and Noor observed different patterns of gene expressions between them, particularly for genes affecting spermatogenesis (Michalak & Noor, 2003). This may be due to the fact that spermatogenesis lacks any postmeiotic transcription regulation and therefore is very sensible to any slight misregulation (Wu & Davis, 1993; Sun et al., 2004). Wu and Davis noted that a delicate balance between X-linked and autosomal genes is required for spermatogenesis, since the X-chromosome follows a particular pattern of condensation during this process, and perturbations in the timing or level of gene expression can cause hybrid sterility (Wu & Davis, 1993). If changes in a few, minor genes is enough to cause strong hybrid sterility, it is not surprising to find numerous genes involved in hybrid sterility among species that diverged long ago and are likely to have accumulated many of those incompatibilities (Wu et al., 1996; Sun et al., 2004). As an example, 120 genes were found to be involved in hybrid sterility between Drosophila simulans and mauritania, which is many more than genes for hybrid female - 20 - sterility or hybrid inviability (Wu et al., 1996). The reasons behind this very fast evolution of male infertility in hybrid offspring is likely to involve the exact same mechanisms as discussed in the previous part of this essay: namely selection for fertilization efficiency and rapid evolution of reproductive features (Wu et al., 1996). If this is the case and if strong selection on spermatogenesis to improve fertilization success results in hybrid incompatibilities, we would expect to find more genes involved in hybrid male sterility in males than in females in taxa with homogametic males as well (i.e. birds and Lepidoptera) (Wu et al., 1996), despite the fact that females might be affected first due to their unbalanced sex chromosomes. Unfortunately, no study has confirmed this prediction among neither birds nor Lepidoptera so far. In the next section I will describe how the Ficedula flycatchers, a model system for the study of speciation, might help us to further understand the patterns of gametic isolation and hybrid sterility in the wild and in birds in particular. 4. A model system for the study of pre- and postzygotic isolation: the Ficedula flycatchers The collared (Ficedula albicollis) and the pied flycatcher (F. hypoleuca) are two closely related passerine species that diverged less than two million years ago and probably went through repeated events of allopatry and secondary contact during the cycles of the last glaciation events in Europe (Saetre et al., 2001; Ellegren et al., 2012). The two species have very similar phenotypes and ecology. The males are black and white, the collared flycatchers have a white collar on their neck and a more pronounced white patch on their forehead (see Figure 4) and are also slightly bigger than the pied flycatcher males, while females of both species are grey-brownish (Vallin et al., 2011). Both species have co-occurred during the reproductive season in a large hybrid zone in central Europe for 12’000 years and more recently on the Baltic islands of Gotland (for 150 years) and Öland (since 1960), in Sweden (Qvarnström et al., 2009). The pied and the collared flycatchers have similar diets and habitat preferences and males of the two species are thus competing over territories at the start of the breeding season (Vallin et al., 2011). Females prefer mates of their own species, but hybridization still occurs and 4% of the breeding pairs on the island of Öland are mixed pairs between collared and pied flycatchers (Qvarnström et al., 2010; Saetre & Saether, 2010). Hybridization is not without consequences, as reflected by the strong postzygotic isolation acting against hybrid offspring. All hybrid females are sterile, following Haldane’s rule (females are the heterogametic sex in birds, see section 3.2), and lay eggs that never hatch. There is strong sexual selection on hybrid males due to their intermediate plumage (see Figure 4) and song and they achieve only 47% of the reproductive success of pure-species males due to their difficulty of finding mates and elevated levels of nestlings issued from extra-pair copulations in their nests (Svedin et al., 2008). Backcross individuals are - 21 - extremely rare and consequently, the fitness of heterospecific pairs calculated over three generations drops to less than 3% of that of conspecific pairs (Wiley et al., 2009). Figure 4: Left: collared flycatcher male, middle: pied flycatcher male, right: hybrid flycatcher male. Several pre-mating barriers help preventing hybridization and facilitate the coexistence of the two species in sympatry. As mentioned before, the males differ in their plumage coloration, but also in their song and females choose their mate accordingly, with a strong preference for conspecific males (Veen et al., 2001). Pied and collared flycatchers have different wintering grounds in Africa and take different migration routes to their breeding areas in the spring (Veen et al., 2007). Both species feed their nestlings caterpillar and thus prefer deciduous forest as breeding sites, but pied flycatchers are more tolerant to harsh conditions and can breed in pine forests, where the food is less abundant (Qvarnström et al., 2009). Males of the two species also differ in their level of aggressiveness: collared flycatchers are more dominant and better at defending territories, resulting in the pied flycatchers being pushed towards fewer, lower quality territories at the Northern tip of the island (Vallin et al., 2012). There is also divergence in breeding time, possibly as a consequence of the differences in habitats and wintering grounds, and pied flycatchers have a tendency to breed later than the collareds (Alatalo et al., 1990). On the other hand, the strong competition between the two flycatcher species enhances the risk of hybridization, since pied flycatcher males have difficulties establishing territories and pied females might be forced to hybridize as a result of the low density of conspecific males (Vallin et al., 2012). Furthermore, some pied flycatchers raised as nestlings in the neighborhood of collared flycatchers include part of the heterospecific song in theirs, which can cause females to fail in recognizing conspecific mates. Indeed, pied flycatcher singing a so-called “mixedsong” have a 30% probability of hybridizing (Haavie et al., 2004; Qvarnström et al., 2006). More recently, studies on the genetics of speciation in the Ficedula flycatchers have demonstrated that both genes coding for characters used in species recognition (e.g. male plumage coloration) and for female preference are situated on the Z chromosome, of which females inherit one copy only from their father (Saether et al., 2007). The recent sequencing and comparison of the flycatcher genomes - 22 - confirms a high divergence of the Z chromosome compared to autosomes, since 35% of all fixed differences between both genomes are found on this chromosome (Ellegren et al., 2012). Meiotic drive was proposed as a major mechanism behind the divergence of the two species, which could have strong consequences on the genetics of hybrid incompatibilities. The mixed population of the hybrid zone on the island of Öland has been monitored in nest-box areas during 1981-1985 and since 2002 and a large amount of data on pre- and post-zygotic ecological processes as well as molecular data have been accumulated. However, the mechanisms involving gametes production and divergence, both at the postmating prezygotic and postzygotic levels, have never been investigated so far. The production of hybrid offspring is very costly and premating reproductive barriers are obviously not completely efficient. Mechanisms allowing females to avoid maladaptive heterospecific fertilization at the gametic level could potentially be very advantageous. Furthermore, the strongly reduced fitness of hybrid males and the low level of introgression observed between species hints towards reduced fertility of hybrid males, which could potentially be observed by analyzing their gametes. As mentioned earlier, the collared and pied flycatcher males differ in levels of aggressiveness and might therefore adopt different mating strategies. Does this result in different levels of extra-pair copulations between species? Does it have an influence on the level of sperm competition between heterospecific males and a consequent divergence in sperm traits between species? Do we observe conspecific sperm precedence when females mate with both conspecifics and heterospecifics? Is there divergence in gamete recognition proteins between both species? Do hybrids have intermediate sperm traits or reproductive strategies that could explain their reduced fitness? These are few of the numerous questions that could potentially be answered by investigating the gametes of the collared and the pied flycatchers in the mixed population of Öland. Preliminary results suggest that there is no major divergence in sperm morphology between species (Podevin, 2011, see Figure 5). However, the levels of extra-pair copulations differ, since 17.2% of collared offspring are issued from extra-pair copulations while there are 22.4% of extra-pair offspring in the nests of pied flycatchers (Podevin, 2011). Despite any obvious divergence in sperm morphology between species, many other mechanisms could contribute to a differential fertilization success of sperm of the two species (conspecific sperm precedence), including differences in the ability of sperm to swim in the reproductive tract of females of the two species and divergences in reproduction proteins influencing sperm transport, storage and/or fertilization success. The analysis of ejaculates of hybrid males collected over the three last years suggest that they are strongly affected by genetic incompatibilities resulting in the absence of sperm production in most hybrid males and the production of immature spermatids in some of them (Figure 5), a sign of spermatogenesis dysfunction (Ålund et al., under review). Moreover, extreme rates of egg hatching failures and extra-pair paternities were observed in the nests of hybrid males, and no hybrid male breeding in the population between 2010 and 2012 sired - 23 - any offspring. Hatching failures could be the result of absence of fertilization as well as early embryonic death due to developmental incompatibilities (Birkhead et al., 2008). The extreme rate of extra-pair paternity (100%) indicates that if any sperm was produced by those males, it was either nonfunctional or performed poorly compared to the sperm of pure-species males. The advance of molecular tools and the recent publication of the flycatcher genome (Ellegren et al., 2012) might allow us to get more insights into the genetic basis of hybrid male incompatibilities between the collared and the pied flycatchers. Figure 5: Left: pied flycatcher sperm, middle: collared flycatcher sperm, right: immature spermatids from a hybrid male. Conclusions In this essay, I gave an overview of the role of gametes as reproductive isolation barriers between heterospecific individuals, both before and after fertilization. We have seen that gametes and reproductive proteins evolve extremely quickly and their divergence constitutes a potentially strong prezygotic isolation barrier, particularly in organisms with external fertilization. Furthermore, competition between sperm of different species and mechanisms of cryptic female choice allow sperm from conspecifics or “preferred” males to be used first for fertilization. Sexual conflict resulting from the different interests of sexes over fertilization might play a strong role in accelerating the evolution of reproductive barriers, through a continuous arm race between adaptations in females and males. When hybridization nevertheless occurs, the next level of reproductive barrier between species is incompatibilities in the hybrid offspring. We have seen that hybrid incompatibilities can arise as a byproduct of genetic divergences in allopatry, and alleles having a positive effect within species can cause dysfunctions when found together in the hybrid genomic background. Hybrid sterility caused by spermatogenesis dysfunctions is pretty common in species with heterogametic males and genes causing hybrid sterility are more numerous than genes for hybrid inviability. - 24 - In general, postmating prezygotic isolation and intrinsic postzygotic incompatibilities are less wellstudied than extrinsic ecological factors and are almost entirely restricted to model or laboratory organisms. Furthermore, the best studied cases of gamete isolation are species with external fertilization while hybrid incompatibilities are studied in taxa with heterogametic males. The Ficedula flycatchers have been studied since decades and still not much is known about gamete interactions between the two hybridizing species and gamete production in the hybrids. They potentially constitute a good model for the study of gamete isolation in the wild in internal fertilizers and for hybrid incompatibilities in homogametic males. The comparison of gametes, reproductive proteins and fertilization patterns between pied, collared and hybrid flycatchers might help us understanding some of the key-questions in the study of speciation. Acknowledgements I would like to thank my supervisor Anna Qvarnström for her useful comments and Johan Ålund for his precious contribution to the graphical parts of this essay. References Alatalo, R. V, Eriksson, D., Gustafsson, L. & Lundberg, A. 1990. Hybridization between Pied and Collared Flycatchers-selection and speciation theory. JEB 3: 375–389. Alcaide, M., Rodríguez, A., Negro, J.J. & Serrano, D. 2012. Male transmission ratio distortion supports MHC-linked cryptic female choice in the lesser kestrel (Aves: Falconidae). Behav. Ecol. Sociobiol. 66: 1467–1473. Arnqvist, G., Edvardsson, M., Friberg, U. & Nilsson, T. 2000. Sexual conflict promotes speciation in insects. PNAS 97: 10460–4. Arnqvist, G. & Rowe, L. 2005. Sexual Conflict, Monographs in behavior and ecology. Princeton University Press. Avise, J.C. 1997. Phylogenetics and the origin of species. PNAS 94: 7748–7755. Barton, N.H. & Hewitt, G.M. 1985. Analysis of hybrid zones. Ann. Rev. Ecol. Syst. 16: 113– 148. - 25 - Barton, N.H. & Hewitt, G.M. 1981. Hybrid zones and speciation. In: Evolution and Speciation: Essays in Honour of M. J. D. White (W. R. Atchley & D. S. Woodruff, eds), pp. 109–145. Cambridge Univ. Press. Berlin, S., Qu, L. & Ellegren, H. 2008. Adaptive evolution of gamete-recognition proteins in birds. J.Mol.Evol. 67: 488–96. Birkhead, T.R. & Brillard, J.P. 2007. Reproductive isolation in birds: postcopulatory prezygotic barriers. TREE 22: 266–72. Birkhead, T.R., Hall, J., Schut, E. & Hemmings, N. 2008. Unhatched eggs: methods for discriminating between infertility and early embryo mortality. Ibis 150: 508–517. Borodin, P.M., Barreiros-Gomez, S.C., Zhelezova, A.I., Bonvicino, C.R. & Andrea, P.S.D. 2006. Reproductive isolation due to the genetic incompatibilities between Thrichomys pachyurus and two subspecies of Thrichomys apereoides ( Rodentia , Echimyidae ). Genome 49: 159–167. Britton-davidian, J., Fel-clair, F., Lopez, J., Alibert, P. & Boursot, P. 2005. Postzygotic isolation between the two European subspecies of the house mouse : estimates from fertility patterns in wild and laboratory-bred hybrids. Biol. J. Linn. Soc. 84: 379–393. Burke, J.M. & Arnold, M.L. 2001. Genetics and the fitness of hybrids. Ann. Rev. genetics 35: 31–52. Charlesworth, B., Coyne, J.A. & Barton, N.H. 1987. The Relative Rates of Evolution of Sex Chromosomes and Autosomes. Am Nat 130: 113–146. Clancy, D.J., Hime, G.R. & Shirras, a D. 2011. Cytoplasmic male sterility in Drosophila melanogaster associated with a mitochondrial CYTB variant. Heredity 107: 374–6. Clark, M.E., O’Hara, F.P., Chawla, a & Werren, J H. 2010. Behavioral and spermatogenic hybrid male breakdown in Nasonia. Heredity 104: 289–301. Clark, N.L., Aagaard, J.E. & Swanson, W.J. 2006. Evolution of reproductive proteins from animals and plants. Reproduction 131: 11–22. Close, R.L., Bell, J.N., Dollin, a E. & Harding, H.R. 1992. Spermatogenesis and synaptonemal complexes of hybrid Petrogale (Marsupialia). Heredity 87: 96–107. Coyne, J.A. & Orr, H.A. 1997. “ Patterns of Speciation in Drosophila ” Revisited. Evolution 51: 295–303. Coyne, J.A. & Orr, H.A. 2004. Speciation. SinauerAssociates Inc. Craft, W.A. 1938. The sex ratio in mules and other hybrid mammals. Quart.Rev. of Biol. 13: 19–40. Crew, F.A.E. & Koller, P.C. 1936. Genetical and cytological studies of the intergeneric hybrid of Cairina moschata and Anas platyrrhynchos. Proc. Roy. Soc. Edinb. 56: 210–292. - 26 - De la Cruz, F. & Davies, J. 2000. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 8: 128–133. Dieckmann, U. & Doebeli, M. 1999. On the origin of species by sympatric speciation. Nature 400: 354–7. Dobzhansky, T. 1936. Studies of hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21: 113–135. Dobzhansky, T. 1934. Studies on hybrid sterility I. Spermatogenesis in pure and hybrid Drosophila pseudoobscura. Z. Zellf. mikr. Anat 21: 169–223. Dobzhansky, Theodosius. 1937. Genetics and the origin of species. Columbia University. Dzur-Gejdosova, M., Simecek, P., Gregorova, S., Bhattacharyya, T. & Forejt, J. 2012. Dissecting the genetic architecture of F1 hybrid sterility in house mice. Evolution no–no. Eberhard, W. 1996. Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press. Ellegren, H., Smeds, L., Burri, R., Olason, P.I., Backström, N., Kawakami, T., et al. 2012. The genomic landscape of species divergence in Ficedula flycatchers. Nature Engqvist, L. 2012. Evolutionary modeling predicts a decrease in postcopulatory sperm viability as a response to increasing levels of sperm competition. Am Nat 179: 667–77. Findlay, G.D. & Swanson, W.J. 2010. Proteomics enhances evolutionary and functional analysis of reproductive proteins. BioEssays 32: 26–36. Frank, S.A. 1991. Divergence of Meiotic Drive-Suppression Systems as an Explanation for Sex- Biased Hybrid Sterility and Inviability. Evolution 45: 262–267. Gadau, J., Page, R.E. & Werren, John H. 1999. Mapping of Hybrid Incompatibility Loci in Nasonia. Genetics 153: 1731–1741. Gavrilets, S. 2004. Fitness Landscapes and the Origin of Species (Monographs in Population Biology). Princeton University Press. Gomendio, M. & Roldan, E.R. 1991. Sperm competition influences sperm size in mammals. Proc. Roy. Soc. B 243: 181–5. Good, J M, Handel, M.A. & Nachman, M W. 2008. Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution 62: 50–65. Good, Jeffrey M, Dean, M.D. & Nachman, Michael W. 2008. A complex genetic basis to Xlinked hybrid male sterility between two species of house mice. Genetics 179: 2213–28. Grant, P.R. & Grant, B.R. 1992. Hybridization of bird species. Science 256: 193–7. - 27 - Grant, P.R., Grant, B.R. & Deutsch, J.C. 1996. Speciation and Hybridization in Island Birds [and Discussion]. Phil. Roy. Soc. B 351: 765–772. Gregory, P.G. & Howard, D.J. 1994. A postinsemination barrier to fertilization isolates two closely related ground crickets. Evolution 48: 705–710. Haavie, J., Borge, T., Bures, S., Garamszegi, L.Z., Lampe, H.M., Moreno, J., et al. 2004. Flycatcher song in allopatry and sympatry - convergence, divergence and reinforcement. JEB 17: 227–237. Haldane, J.B.S. 1922. Sex Ratio and Unisexual Sterility in hybrid Animals. J. Genet. XII: 101–109. Helfenstein, F., Podevin, M. & Richner, H. 2009. Sperm morphology, swimming velocity, and longevity in the house sparrow Passer domesticus. Behav. Ecol. Sociobiol. 64: 557– 565. Hewitt, G.M., Mason, P. & Nichols, R. a. 1989. Sperm precedence and homogamy across a hybrid zone in the alpine grasshopper Podisma pedestris. Heredity 62: 343–353. Howard, D.J. 1999. Conspecific Sperm and Pollen Precedence and Speciation. Ann. Rev. Ecol. Syst. 30: 109–132. Howard, D.J., Gregory, P.G., Chu, J. & Cain, M.L. 1998. Conspecific Sperm Precedence is an Effective Barrier to Hybridization Between Closely Related Species. Evolution 52: 511– 516. Howard, D.J., Palumbi, S.R., Birge, L.M. & Manier, M.K. 2009. Sperm and speciation. In: Sperm biology: an evolutionary perspective, pp. 367–403. Immler, S., Hamilton, M.B., Poslusny, N.J., Birkhead, T.R. & Epifanio, J.M. 2011. Postmating reproductive barriers in two unidirectionally hybridizing sunfish (Centrarchidae: Lepomis). JEB 24: 111–120. Immler, S., Pryke, S.R., Birkhead, T.R. & Griffith, S.C. 2010. Pronounced within-individual plasticity in sperm morphometry across social environments. Evolution 64: 1634–43. Johnsgard, P.A. 1970. A Summary of Intergeneric New World Quail Hybrids, and a New Intergeneric Hybrid Combination. The Condor 72: 85–88. Kopp, A. & Frank, A.K. 2005. Speciation in progress? A continuum of reproductive isolation in Drosophila bipectinata. Genetica 125: 55–68. Landry, C., Geyer, L.B., Arakaki, Y., Uehara, T. & Palumbi, S.R. 2003. Recent speciation in the Indo-West Pacific: rapid evolution of gamete recognition and sperm morphology in cryptic species of sea urchin. Proc. Roy. Soc. B 270: 1839–47. Lijtmaer, D. a, Mahler, B. & Tubaro, P.L. 2003. Hybridization and postzygotic isolation patterns in pigeons and doves. Evolution 57: 1411–8. - 28 - Lillie, F.R. 1921. Studies of fertilization VIII. On the measure of specificity in fertilization between two associated species of the sea-urchin genus Strongylocentrotus. Biol. Bull. 40: 1–22. Ludlow, a M. & Magurran, a E. 2006. Gametic isolation in guppies (Poecilia reticulata). Proc. Roy. Soc. B 273: 2477–82. Mallet, J. 2005. Hybridization as an invasion of the genome. TREE 20: 229–37. Manwell, C. & Baker, C.M.A. 1970. Molecular biology and the origin of species: heterosis, protein polymorphism and animal breeding. London: Sidgwick & Jackson. Marshall, J.L., Huestis, D.L., Garcia, C., Hiromasa, Y., Wheeler, S., Noh, S., et al. 2011. Comparative proteomics uncovers the signature of natural selection acting on the ejaculate proteomes of two cricket species isolated by postmating, prezygotic phenotypes. Mol. Biol. Evol. 28: 423–435. Martín-Coello, J., Benavent-Corai, J., Roldan, E.R. & Gomendio, M. 2009. Sperm competition promotes asymmetries in reproductive barriers between closely related species. Evolution 63: 613–23. Matute, D.R. 2010. Reinforcement of gametic isolation in Drosophila. PLoS biol 8: e1000341. Mayr, E. 1942. Systematics and the Origin of Species, from the Viewpoint of a Zoologist. Harvard University Press. McDermott, S.R. & Noor, M.A.F. 2010. The role of meiotic drive in hybrid male sterility. Phil. Trans. R. Soc. B 365: 1265–1272. Michalak, P. & Noor, M.A.F. 2003. Genome-wide patterns of expression in Drosophila pure species and hybrid males. Mol. Biol. Evol. 20: 1070–6. Miller, R.L. 1997. Specificity of sperm chemotaxis among great barrier reef shallow-water Holothurians and Ophiuroids. J. Exp. Zool. 279: 189–200. Moehring, A.J. 2011. Heterozygosity and Its Unexpected Correlations With Hybrid Sterility. Evolution 65(9) 2621-30. Muller, H.J. & Pontecarvo, G. 1942. Recessive genes causing interspecific sterility and other disharmonies between Drosophila melanogaster and simulans. Genetics 27: 157. Müller, H.J. 1940. Bearing of the Drosophila work on systematics. In: The New Systematic (J. S. Huxley, ed), pp. 185–268. Clarendon Press, Oxford. Nakano, S. 1985. Effect of Interspecific Mating on Female Fitness in Two Closely Related Ladybirds (Henosepilachna). Jap. j. entomol. 53: 112–119. - 29 - Oka, A., Mita, A., Takada, Y., Koseki, H. & Shiroishi, T. 2010. Reproductive isolation in hybrid mice due to spermatogenesis defects at three meiotic stages. Genetics 186: 339– 51. Orr, H.A. 2005. The genetic basis of reproductive isolation: insights from Drosophila. PNAS 102 Suppl: 6522–6. Orr, H.A. 1995. The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics 139: 1805–13. Palumbi, S.R. 2009. Speciation and the evolution of gamete recognition genes: pattern and process. Heredity 102: 66–76. Parker, G.A. 1979. Sexual selection and sexual conflict. In: Sexual selection and reproductive competition in insects (M. S.Blum and N. A. Blum, ed), pp. 123–166. Academic, New York. Parker, G.A. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45: 525–567. Phadnis, N. & Orr, H.A. 2009. a single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science 323: 376–379. Pitnick, S., Wolfner, M.F. & Suarez, S.S. 2009. Ejaculate-female and sperm-female interactions. In: Sperm biology: an evolutionary perspective, pp. 247–304. Pizzari, T. & Parker, G.A. 2009. Sperm competition and sperm phenotype. In: Sperm biology: an evolutionary perspective, pp. 207–245. Podevin, M. 2011. Sperm morphology and reproductive isolation in Ficedula flycatchers, master thesis, Uppsala University. Presgraves, D.C. 2002. Patterns of postzygotic isolation in Lepidoptera. Evolution 56: 1168– 83. Presgraves, D.C. 2010. The molecular evolutionary basis of species formation. Nat. rev. Genet. 11: 175–80. Presgraves, D.C. & Orr, H.A. 1998. Haldane’s Rule in Taxa Lacking a Hemizygous X. Science 282: 952–954. Price, C.S.C., Kim, C.H., Gronlund, C.J. & Coyne, J.A. 2001. Cryptic reproductive isolation in the Drosophila simulans species complex. Evolution 55: 81–92. Price, C.S.C., Kim, C.H., Posluszny, J. & Coyne, J.A. 2000. Mechanisms of conspecific sperm precedence in Drosophila. Evolution 54: 2028–37. Price, T.D. & Bouvier, M.M. 2002. The evolution of F1 postzygotic incompatibilities in birds. Evolution 56: 2083–9. - 30 - Qvarnström, A., Haavie, J., Saether, S.A., Eriksson, D. & Pärt, T. 2006. Song similarity predicts hybridization in flycatchers. JEB 19: 1202–9. Qvarnström, A., Rice, A.M. & Ellegren, H. 2010. Speciation in Ficedula flycatchers. Proc. Roy. Soc. B 365: 1841–52. Qvarnström, A., Wiley, C., Svedin, N. & Vallin, N. 2009. Life-history divergence facilitates regional coexistence of competing Ficedula flycatchers. Ecology 90: 1948–57. Reed, L.K. & Markow, T.A. 2004. Early events in speciation: polymorphism for hybrid male sterility in Drosophila. PNAS 101: 9009–12. Reifová, R., Kverek, P. & Reif, J. 2011. The first record of a female hybrid between the Common Nightingale (Luscinia megarhynchos) and the Thrush Nightingale (Luscinia luscinia) in nature. J. Ornith. 152: 1063–1068. Rhymer, J.M. & Simberloff, D. 1996. Extinction By Hybridization and Introgression. Ann. Rev. Ecol. Syst. 27: 83–109. Rice, A.M., Rudh, A., Ellegren, H. & Qvarnström, A. 2011. A guide to the genomics of ecological speciation in natural animal populations. Ecol. Let. 14: 9–18. Rigdon, R.H. & Mott, C. 1965. Testis in the sterile hybrid duck: a histologic and hisochemical study. Pathologia Veterinaria 2: 553–565. Saether, S.A., Saetre, G.-P., Borge, T., Wiley, C., Svedin, N., Andersson, G., et al. 2007. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318: 95–7. Saetre, G.-P., Borge, T., Lindell, J., Moum, T., Primmer, C., Sheldon, B.C., et al. 2001. Speciation, introgressive hybridization and nonlinear rate of molecular evolution in flycatchers. Mol. Ecol. 10: 737–49. Saetre, G.-P. & Saether, S.A. 2010. Ecology and genetics of speciation in Ficedula flycatchers. Mol. Ecol. 19: 1091–106. Sagga, N. & Civetta, A. 2011. Male-Female Interactions and the Evolution of Postmating Prezygotic Reproductive Isolation among Species of the Virilis Subgroup. Int. J. Evol. Biol. 2011: 485460. Schluter, D. 2001. Ecology and the origin of species. TREE 16: 372–380. Servedio, M.R. 2001. Beyond reinforcement: the evolution of premating isolation by direct selection on preferences and postmating, prezygotic incompatibilities. Evolution 55: 1909–1920. Servedio, M.R., Van Doorn, G.S., Kopp, M., Frame, A.M. & Nosil, P. 2011. Magic traits in speciation: “magic” but not rare? TREE 26: 389–397. - 31 - Sun, S., Ting, C.T. & Wu, C.I. 2004. The normal function of a speciation gene, Odysseus, and its hybrid sterility effect. Science 305: 81–3. Svedin, N., Wiley, C., Veen, T., Gustafsson, L. & Qvarnström, A. 2008. Natural and sexual selection against hybrid flycatchers. Proc. Roy. Soc. B 275: 735–44. Swanson, W.J. & Vacquier, V.D. 2002a. Reproductive Protein Evolution. Ann. Rev. Ecol. Syst. 33: 161–179. Swanson, W.J. & Vacquier, V.D. 2002b. The rapid evolution of reroductive proteins. Genetics 3: 137–144. Takahashi, H., Nagai, T. & Goto, A. 2005. Hybrid male sterility between the fresh- and brackish-water types of ninespine stickleback Pungitius pungitius (Pisces, Gasterosteidae). Zool. science 22: 35–40. Tao, Y., Chen, S., Hartl, D.L. & Laurie, C.C. 2003. Genetic Dissection of Hybrid Incompatibilities Between Drosophila simulans and D. mauritiana. I. Differential Accumulation of Hybrid Male Sterility Effects on the X and Autosomes. Genetics 164: 1383–1397. The Marie Curie Speciation Network. 2012. What do we need to know about speciation? TREE 27: 27–39. Turelli, M., Barton, N.H. & Coyne, J.A. 2001. Theory and speciation. TREE 16: 330–343. Turner, L.M. & Hoekstra, H.E. 2008. Causes and consequences of the evolution of reproductive proteins. Int. J. Dev. Biol. 52: 769–80. Vacquier, V.D. 1998. Evolution of Gamete Recognition Proteins. Science 281: 1995–1998. Vallin, N., Rice, A.M., Arntsen, H., Kulma, K. & Qvarnström, A. 2011. Combined effects of interspecific competition and hybridization impede local coexistence of Ficedula flycatchers. Evol. Ecol. 26: 927–942. Vallin, N., Rice, A.M., Bailey, R.I., Husby, A. & Qvarnström, A. 2012. Positive feedback between ecological and reproductive character displacement in a young avian hybrid zone. Evolution 66: 1167–79. Van Doorn, G.S., Edelaar, P. & Weissing, F.J. 2009. On the origin of species by natural and sexual selection. Science 326: 1704–7. Veen, T., Borge, T., Griffith, S.C., Saetre, G.-P., Bures, S., Gustafsson, L., et al. 2001. Hybridization and adaptive mate choice in flycatchers. Nature 411: 45–50. Veen, T., Svedin, N., Forsman, J.T., Hjernquist, M.B., Qvarnström, A., Hjernquist, K.A.T., et al. 2007. Does migration of hybrids contribute to post-zygotic isolation in flycatchers? Proc. Roy. Soc. B 274: 707–12. - 32 - Wade, M.J., Patterson, H., Chang, N.W. & Johnson, N. a. 1994. Postcopulatory, prezygotic isolation in flour beetles. Heredity 72 ( Pt 2): 163–7. Watson, E.T. & Demuth, J.P. 2012. Haldane’s Rule in Marsupials: What Happens When Both Sexes Are Functionally Hemizygous? heredity 103: 453–458. Wiley, C., Qvarnström, A., Andersson, G., Borge, T. & Saetre, G.-P. 2009. Postzygotic isolation over multiple generations of hybrid descendents in a natural hybrid zone: how well do single-generation estimates reflect reproductive isolation? Evolution 63: 1731–9. Wittbrodt, J., Adam, D., Malitschek, B., Mäueler, W., Raulf, F., Telling, A., et al. 1989. Novel putative receptor tyrosine kinase encoded by the melanoma-inducing Tu locus in Xiphophorus. Nature 341: 415–21. Wu, C.I. & Davis, A.W. 1993. Evolution of Postmating Reproductive Isolation : The Composite Nature of Haldane ’ s Rule and Its Genetic Bases. Am. Nat. 142: 187–212. Wu, C.I., Johnson, N.A. & Palopoli, M.F. 1996. Haldane’s rule and its legacy: why are there so many sterile males? TREE 11: 281–284. Yamashina, M.Y. 1943. Studies on Sterility in Hybrid BirdsⅣ. Cytological researches on hybrids in the family phasianidae. J. Fac. Sci., Hokkaido Imperial University, ser. 6 8: 307–386. Zeh, J. & Zeh, D.W. 1997. The evolution of polyandry II: post-copulatory defenses against genetic incompatibility. Proc. Roy. Soc. B 264: 69–75. - 33 - ESSAYS IN THE SERIES 1 Reproductive patterns in marine animals on shallow bottoms. Anne-Marie Edlund. 1979 2 Production in estuaries and saltmarshes. Staffan Thorman. 1979 3 The evolution of life history traits. Anders Berglund. 1979 4 Predation in marine littoral communities. Carin Magnhagen. 1979 5 Insular biogeographic theory, developments in the past decade. Stefan Åhs. 1979 6 Theories and tests of optimal diets. Juan Moreno. 1979 7 Ecological characters in insular communities. Mats Malmquist. 1980 8 Plant/insect relationships: a synopsis. Ola Jennersten. 1980 9 Sexual size dimorphism in birds of prey. Per Widén. 1980 10 The evolution of avian mating systems. Per Angelstam. 1980 11 Dispersal, dispersion and distribution in small rodent populations. Fredric Karlsson. 1980 12 Age-related differences of competence in birds. Karin Ståhlbrandt. 1980 13 Respiration measurements as a tool in fish ecology. Bengt Fladvad. 1981 14 Intraspecific variation; its adaptive value and consequences. Lars Gustafsson. 1981 15 Cooperative breeding in birds: theories and facts. Mats Björklund. 1982 16 Some aspects of parasitism. Göran Sundmark. 1982 17 Evolution and ecology of migration and dispersal. Jan Bengtsson. 1982 18 Are animals lazy? - Time budgets in ecology. Bodil Enoksson. 1983 19 Ecology of island colonization. Torbjörn Ebenhard. 1983 20 Reproductive habits and parental care in some lacustrine African Cichlids (Pisces) with special reference to Lake Malawi species. Ulrich Jessen. 1983 21 Monogamy or polygyny? - Mating systems in passerine birds. Björn Westman. 1983 22 Distributional patterns in reptiles and amphibians. Per Sjögren. 1983 23 Some theoretical considerations of dispersal. Gunnar Nilsson. 1983 24 Interactions between animals and seeds. Urban Wästljung. 1984 25 Reproductive modes and parental care in fishes. Ingrid Svensson. 1984 26 Reproductive strategies in fishes. Peter Karås. 1984 27 Sexual selection and female choice based on male genetic qualities. Jacob Höglund. 1984 28 The timing of breeding in birds with special reference to the proximate control. Mats Lindén. 1985 29 Philopatry and inbreeding versus dispersal and outbreeding. Tomas Pärt. 1985 30 Evolution of sociality in Hymenoptera. Folke Larsson. 1985 31 Bird song and sexual selection. Dag Eriksson. 1986 32 The energetics of avian breeding. Lars Hillström. 1986 33 Sex role reversal in animals. Gunilla Rosenqvist. 1986 34 Factors affecting post-fledging survival in birds. Kjell Larsson. 1986 35 Quantitative genetics in evolutionary ecology: methods and applications. Pär Forslund. 1986 36 Body size variations in small mammals. Anders Forsman. 1986 37 Habitat heterogeneity and landscape fragmentation - consequences for the dynamics of populations. Henrik Andrén. 1986 38 The evolution of predispersal seed predation systems in insects. Mats W. Pettersson. 1987 39 Animal aggregations. Anette Baur. 1988 40 Operational and other sex ratios: consequences for reproductive behaviour, mating systems and sexual selection in birds. Jan Sundberg. 1988 41 Genetic variation in natural populations. Annika Robertson. 1989 42 Adaptations for a parasitic way of life. Reija Dufva. 1989 43 Predator-prey coevolution. Lars Erik Lindell. 1990 44 Parasite impact on host biology. Klas Allander. 1990 45 Ecological factors determining the geographical distribution of animal populations. Berit Martinsson. 1990 46 Mate choice - mechanisms and decision rules. Elisabet Forsgren. 1990 47 Sexual selection and mate choice with reference to lekking behaviour. Fredrik Widemo. 1991 48 Nest predation and nest type in passerine birds. Karin Olsson. 1991 49 Sexual selection, costs of reproduction and operational sex ratio. Charlotta Kvarnemo. 1991 50 Evolutionary constraints. Juha Merilä. 1991 51 Variability in the mating systems of parasitic birds. Phoebe Barnard. 1993. ISRN UU-ZEK-IRE--51--SE 52 Foraging theory: static and dynamic optimization. Mats Eriksson. 1993. ISRN UU-ZEK-IRE--52--SE 53 Moult patterns in passerine bird species. Christer Larsson. 1993. ISRN UU-ZEK-IRE--53--SE 54 Intraspecific variation in mammalian mating systems. Cheryl Jones. 1993. ISRN UU-ZEK-IRE--54--SE 55 The Evolution of Colour Patterns in Birds. Anna Qvarnström. 1993. ISRN UU-ZEK-IRE--55--SE 56 Habitat fragmentation and connectivity. Torbjörn Nilsson. 1993. ISRN UU-ZEK-IRE--56--SE 57 Sexual Selection. Models, Constraints and Measures. David Stenström. 1994. ISRN UU-ZEK-IRE--57--SE 58 Cultural Transmission and the Formation of Traditions in Animals. Henk van der Jeugd. 1995. ISRN UU-ZEK-IRE--58—SE 59 Ecological Factors Affecting the Maintenance of Colour Polymorphism. Sami Merilaita. 1995. ISRN UU-ZEK-IRE--59--SE 60 The Evolution and Maintenance of Hermaphroditism in Animals. Anna Karlsson. 1996. ISRN UU-ZEK-IRE--60--SE 61 Costs and Benefits of Coloniality in Birds. Tomas Johansson. 1996. ISRN UU-ZEK-IRE--61--SE 62 Nutrient reserve dynamics in birds. Måns S. Andersson. 1996. ISRN UU-ZEK-IRE--62--SE 63 Nest-building for parental care, mate choice and protection in fishes. Sara Östlund. 1996. ISRN UU-ZEK-IRE--63--SE 64 Immunological ecology. Dag Nordling. 1996. ISRN UU-ZEK-IRE--64--SE 65 Hybrid zones and speciation by reinforcement. Anders Ödeen. 1996. ISRN UU-ZEK-IRE--65--SE 66 The timing of breeding onset in temperate zone birds. Robert Przybylo. 1996. ISRN UU-ZEK-IRE--66--SE 67 Founder speciation - a reasonable scenario or a highly unlikely event? Ann-Britt Florin. 1997. ISRN UU-ZEK-IRE--67--SE 68 Effects of the Social System on Genetic Structure in Mammal Populations. Göran Spong. 1997. ISRN UU-ZEK-IRE--68--SE 69 Male Emancipation and Mating Systems in Birds. Lisa Shorey. 1998. ISRN UU-ZEK-IRE--69--SE 70 Social behaviours as constraints in mate choice. Maria Sandvik. 1998. ISRN UU-ZEK-IRE--70--SE 71 Social monogamy in birds. Kalev Rattiste. 1999. ISRN UU-ZEK-IRE--71--SE 72 Ecological determinants of effective population size. Eevi Karvonen. 2000. ISRN UU-ZEK-IRE--72--SE 73 Ecology of animals in ephemeral habitats. Jonas Victorsson. 2001. ISRN UU-ZEK-IRE--73--SE 74 Extra-pair paternity in monogamous birds. Katherine Thuman. 2001. ISRN UU-ZEK-IRE--74-SE 75 Intersexual communication in fish. Niclas Kolm. 2001. ISRN UU-ZEK-IRE--75--SE 76 The role of postmating prezygotic reproductive isolation in speciation. Claudia Fricke. 2002. ISRN UU-ZEK-IRE--76--SE 77 Host parasite interactions: Local adaptation of parasites and changes in host behaviour. Lena Sivars. 2002. ISRN UU-ZEK-IRE--77—SE 78 The molecular clock hypothesis. Reliable story or fairy tale? Marta Vila-Taboada. 2002. ISRN UU-ZEK-IRE--78--SE 79 Chemical cues in mate choice. Björn Johansson. 2002. ISRN UU-ZEK-IRE--79--SE 80 Does sexual selection promote speciation? A comparative analysis of the Family Labridae (wrasses). Sarah Robinson. 2002. ISRN UU-ZEK-IRE--80--SE 81 Population bottlenecks and their effects on genetic diversity: introducing some recent methods for detection. Marnie H Demandt. 2003. ISRN UU-ZEK-IRE--1--E 82 Immune response and its correlations to other traits in poultry. Joanna Sendecka. 2003. ISRN UU-ZEK-IRE--82--SE 83 Resistance of Hybrids to Parasites. Chris Wiley. 2003. ISRN UU-ZEK-IRE--83--SE 84 The Role of Genetics in Population Viability Analysis. Johanna Arrendal. 2003. ISRN UU-ZEK-IRE--84--SE 85 Sexual Selection and Speciation – The Scent of Compatibility. Nina Svedin. 2003. ISRN UU-ZEK-IRE--85--SE 86 Male-female coevolution: patterns and process. Johanna Rönn. 2004. ISRN UU-ZEK-IRE--86—SE 87 Inbreeding in wild populations and environmental interactions. Mårten Hjernquist. 2004. ISRN UU-ZEK-IRE--87—SE 88 Sympatric speciation – A topic of no consensus. Emma Rova. 2005. ISRN UU-ZEK-IRE--88--SE 89 Man-made offshore installations: Are marine colonisers a problem or an advantage? Olivia Langhamer. 2005. ISRN UU-ZEK-IRE--89--SE 90 “Isolation by distance”: – A biological fact or just a theoretical model? Sara Bergek. 2006. ISRN UU-ZEK-IRE--90--SE 91 Signal evolution via sexual selection – with special reference to Sabethine mosquitoes. Sandra South. 2007. ISRN UU-ZEK-IRE--91--SE 92 Aposematic Colouration and Speciation – An Anuran Perspective. Andreas Rudh. 2008. ISRN UU-ZEK-IRE--92--SE 93 Models of evolutionary change. Lára R. Hallson. 2008. ISRN UU-ZEK-IRE--93--SE 94 Ecological and evolutionary implications of hybridization. Nicals Vallin. 2009. ISRN UU-ZEK-IRE--94--SE 95 Intralocus sexual conflict for beginners. Paolo Innocenti. 2009. ISRN UU-ZEK-IRE--95--SE 96 The evolution of animal mating systems. Josefin Sundin. 2009. ISRN UU-ZEK-IRE--96--SE 97 The evolutionary ecology of host-parasite interactions. Katarzyna Kulma. 2011. ISRN UU-ZEK-IRE--97--SE 98 Sensory exploitation – trends, mechanisms and implications. Mirjam Amcoff. 2011. ISRN UU-ZEK-IRE--98--SE 99 Evolutionary biology of aging. Hwei-yen Chen 2012. ISRN UU-ZEK-IRE--99--SE