Chemistry Reference Tables
advertisement

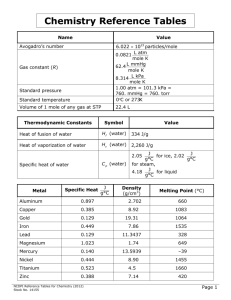
PHYSICAL CONSTANTS AND CONVERSION FACTORS NAME SYMBOL VALUE (with units) Length Meter m Nanometer nm o Angstrom A −9 1 × 10 m −10 1 × 10 m Mass (m) Kilogram kg Atomic Mass Units amu or u 1.66 ´ 10 —24 g Chemistry Reference Tables Volume (V) Liter L 1 mL = 1 cm3 Pressure (P) Standard Pressure 1 atm 1 atm = 101.3 kPa = 760. Torr = 760. mmHg = 14.7 lb/ in2 Temperature (T) Standard Temperature °C 32.0°F = 0.0°C = 273.15K Q 1 cal = 4.184 J Energy Heat 1 Cal = 4.184 kJ Activation Energy Ea kJ/mol Specific Heat of water (l) Cp 4.18 J/g•°C Specific Heat of water (g) Cp 2.02 J/g•°C Specific Heat of water (s) Cp 2.05 J/g•°C Heat of fusion of water ∆Hfus 6.01 kJ/mol or 334 J/g Heat of vaporization ∆Hvap of water 40.7 kJ/mol or 2260 J/g Constants Speed of light c 3.00 × 108 m/s Avogadro’s number NA 6.022 × 10 Charge of an electron e 1.60 × 10 Equilibrium constant K Rate constant k Reaction quotient Q Universal gas constant R 23 –19 particles/mol C 0.08206 L-atm/mol K 62.4 L mmHg/mol K 8.314 J/K mol 8.314 L kPa/mol K 1.99 cal/K mol NCDPI Reference Tables for Chemistry (adopted 2000) i NAME SYMBOL DENSITY BOILING MELTING POINT (K)POINT (K) Ammonia Carbon Dioxide Carbon Monoxide Chlorine Ethanol Glucose Gold Hexane Hydrogen Hydrogen Chloride Hydrogen Sulfide Iron Lead Magnesium Methane Methanol Nitrogen Nitrogen(II) Oxide Oxygen Silver Sodium Bicarbonate Sodium Carbonate Sodium Chloride Sucrose Sulfur Dioxide Tin Water NH3 CO2 CO Cl2 CH3CH2OH C6H12O6 Au C6H14 H2 HCl H2S Fe Pb Mg CH4 CH3OH N2 NO O2 Ag NaHCO3 Na2CO3 NaCl C12H22O11 SO2 Sn H2O 0.771 g/L@STP 1.98 g/L@STP 1.25 g/L@STP 3.21 g/L@STP 0.7893 g/cm3 1.54 g/cm3 19.31 g/cm3 0.6603 g/cm3 0.0899 g/L@STP 1.64 g/L@STP 1.54 g/L@STP 7.86 g/cm3 11.3437 g/cm3 1.74 g/cm3 0.716 g/cm3 0.7914 g/cm3 1.25 g/L@STP 1.34 g/L@STP 1.43 g/L@STP 10.5 g/cm3 2.159 g/cm3 2.532 g/cm3 2.165 g/cm3 1.27 g/cm3 2.92 g/L@STP 5.75 g/cm3 1.00 g/cm3 240 195 subl 82 238 351.5 decompose 3353 342 20 188 212 3023 2013 1380 109 338 77 121 90 2485 decompose decompose 1686 decompose 263 2543 373 195.3 216.4 74 172.02 155.7 359 1337.43 178 13.86 158.2 187.5 1808 600.502 921.8 91 179.1 63.14 109.4 54.6 1234.93 543 1124 1074 359 200.3 504.96 273 STANDARD UNITS Symbol m kg Pa K mol J s C V L ii Name meter kilogram pascal Kelvin mole joule second coulomb volt liter Quantity length mass pressure temperature amount of a substance energy, work, quantity of heat time electric charge electric potential volume NCDPI Reference Tables for Chemistry (adopted 2000) WRITING CHEMICAL EQUATIONS Writing correct chemical equations requires that you know how to predict products of reactions. Even with limited experience, one can use a few guidelines to accomplish this. Seven frequently used elements naturally occur as diatomic molecules: H2, O2, N2, F2, Cl2, Br2, I2. This is how they should always be written in a chemical equation. States of matter should be indicated by (s), (l), or (g) and ions in aqueous solution as (aq). General Classification of Reactions (A, B, C, D represent elements and M represents a metal) 1. Synthesis: A + B → AB 3. Single Replacement: A + BC → AC + B a. Reaction between hydrogen and a nonmetal H2 ( g) + Cl2 ( g) → 2HCl ( g) a. Metal-metal replacement 2Al (s) + 3Fe(NO3)2 (aq) → 2Al(NO3)3 (aq) + 3Fe (s) b. Metal-Nonmetal reactions 2Na (s) + Cl2 ( g) → 2NaCl (s) b. Active metal + water reactions 2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 ( g) c. Metal oxide-water reactions CaO (s) + H2O (l) → Ca(OH)2 (s) c. Metal-Acid reactions 2HCl (aq) + Mg (s) → MgCl2 (aq) + H2 ( g) d. Nonmetal oxide-water reactions SO2 ( g) + H2O (l) → H2SO3 (aq) d. Halide-Halide replacement Cl2 ( g) + 2HBr (aq) → 2HCl (aq) + Br2 (l) 2. Decomposition 4. Double Replacement: a. Binary compounds AB-heat /electricity → A + B 2HgO (s) + heat → 2Hg (l) + O2 ( g) a. Formation of a precipitate from solution Pb(NO3)2 (aq) + 2KI (aq) → PbI2 (s) + 2KNO3 (aq) b. Metallic carbonates MCO3 → MO + CO2 CaCO3 (s) + heat → CaO (s) + CO2 ( g) b. Formation of a gas CaCO3 (s) + 2HCl (aq) → CaCl2 (aq) + H2O (l) + CO2 ( g) AB + CD → AD + BC c. Metallic hydrogen carbonates MHCO3 → MO + H2O + CO2 c. Acid-Base Neutralization Reaction 2NaHCO3 (s) + heat → Na2O (s) + H2O ( g) + 2CO2 ( g) HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) d. Metallic hydroxides MOH → MO + H2O 2NaOH (s) + heat → Na2O (s) + H2O ( g) e. Metallic chlorates decompose MClO3 → MCl + O2 2KClO3 (s) + heat → 2KCl (s) + 3O2 ( g) 5. Combustion Reaction Hydrocarbon + oxygen → Carbon dioxide and water CH4 ( g) + 2O2 ( g) → CO2 ( g) + 2H2O (l) f. Some acids decompose to nonmetal oxides and water H2CO3 (aq) + heat → H2O (l) + CO2 ( g) g. Hydrate decomposition AB • xH2O → AB + xH2O CuSO4 • 5H2O (s) + heat → CuSO4 (s) + 5H2O (l) h. Peroxide 2H2O2 (l) + heat or catalyst → 2H2O (l) + O2 ( g) NCDPI Reference Tables for Chemistry (adopted 2000) iii Bohr Model for Hydrogen Atom n=6 n=5 n=4 n=3 n=2 n=1 -12 10 Gamma ray -10 10 -9 10 -8 -7 10 10 1875 1282 -6 10 -5 10 -4 10 -3 -2 10 X ray 10 -1 10 0 10 1 10 2 10 3 10 4 10 100,000 10,000 1,000 100 10 1.0 0.1 0.01 0.001 0.0001 0.00001 0.000001 0.0000001 0.00000001 0.000000001 0.0000000001 -11 10 IR 5 10 Microwaves UV Visible -13 10 Visible Electromagnetic Spectrum 0.00000000001 0.000000000001 (m) 0.0000000000001 UV (nm) 1094 103 410 434 486 656 97 122 UV = Ultraviolet IR = Infrared IR Radio waves iv Orange 5.9´10 Red 7.0´10 -7 Yellow -7 Green 5.7´10 -7 Blue 4.9´10 -7 4.2´10 -7 4.0´10 -7 Violet (m) Longer Wavelength Visible Light 6.5´10 -7 Shorter Wavelength NCDPI Reference Tables for Chemistry (adopted 2000) 150 Solubility (grams of solute per 100 grams of water) NCDPI Reference Tables for Chemistry (adopted 2000) Solubility Curve 140 URANIUM DISINTEGRATION SERIES gases KI solids 130 100 88 90 92 60 230 226 NH4Cl NH3 Mass 222 Number KCl 218 Na2SO4 NaCl 214 KClO3 210 20 10 0 238 234 HCl 70 30 86 KNO3 80 40 84 NaNO3 90 50 82 Pb Bi Po At Rn Fr Ra Ac Th Pa U 120 110 Atomic Number and Chemical Symbol 206 SO2 10 20 30 40 50 60 70 80 90 100 Temperature (°C) 4 2 He 0e 1 (a particle) Helium nucleus emission (b particle) electron emission v vi D = density m = mass m D = V V = volume K =°C + 273 K = Kelvin V1P1 VP = 2 2 T1 T2 T = temperature Pt = P1 + P2 + P3 + . . . V = volume PV = nRT R = universal gas constant v = rate of effusion mw2 v1 = v2 M = P = pressure mw = molecular mass mw1 moles of solute liters of solution M = molarity n = number of moles of ions nM1V1 = nM2V2 Q = quantity of heat ∆T = change in temperature Q = mCp∆T Cp = specific heat Q = mHf Hf = heat of fusion Hv = heat of vaporization m = mass Q = mHv c = λυ NCDPI Reference Tables for Chemistry (adopted 2000) c = speed of light λ = wavelength υ = frequency E = energy E = hυ h = Planck’s constant [ ] K pOH = − log[OH ] K = [ H ][OH ] = 1 × 10 pH = − log H + − + − w = equilibrium constant for the ionization of water −14 w 0 0 Error = Accepted value – Experimental value × 100 Accepted value SOLUBILITY RULES: SOLUBLE: All Nitrates, Acetates, Ammonium and Group I salts All Chlorides, Bromides, and Iodides, except Silver, Lead, and Mercury(I) All Fluorides except Group II, Lead(II), and Iron(III) All Sulfates except Calcium, Strontium, Barium, Mercury, Lead(II), and Silver INSOLUBLE: All Carbonates and Phosphates except Group I and Ammonium All Hydroxides except Group I, Strontium, and Barium All Sulfides except Group I, II, and Ammonium All Oxides except Group I INSOLUBLE means a precipitate forms when equal volumes of 0.10 M solutions or greater are mixed ACTIVITY SERIES of METALS: Li Rb K Ba Sr Ca Replace hydrogen from cold water Na Activity of Halogens Mg F2 Al Cl2 Mn Br2 Zn I2 Cr Fe Replace hydrogen from steam Cd Co Ni Sn Pb Replace hydrogen from acids [H2] Sb Bi Cu React with oxygen to form oxides Hg Ag Pt Au 1. Any metal higher in the activity series will displace another metal in a single displacement reaction. 2. Metals above water may react with water rather than a metal compound. Half-Reaction H 2 O 2 +2H +2e → 2H 2 O + – PbO 2 +4H + +SO 4 2– + 2e – → PbSO 4 +2H 2 O E° (V) 1.78 1.69 M nO +4H +3e → M nO 2 +2H 2 O 1.68 M nO +8H +5e → M n +4H 2 O 1.51 PbO 2 + 4H + 2e → Pb +2H 2 O 1.46 Cl 2 +2e → 2Cl 1.36 – 4 + – 4 – + – + 2+ – – 2+ – O 2 +4H + +4e – → 2H 2 O 1.23 Br 2 +2e → 2Br 1.09 – – NO 3– +4H + +3e – → NO +2H 2 O 0.96 Ag + +e – → Ag 0.80 I 2 +2e → 2I 0.54 – – Cu +e → Cu + – 0.52 O 2 +2H 2 O+4e → 4OH – – Hg 2 Cl 2 +2e → 2Hg+2Cl – 0.40 – 0.34 Cu 2+ +2e – → Cu 0.34 SO +4H +2e → H 2SO 3 +H 2 O 0.20 Cu 2+ +e – → Cu + 0.16 2H +2e → H 2 0.00 Fe +3e → Fe –0.036 Pb +2e → Pb –0.13 Sn +2e → Sn –0.14 2– 4 + + – – 3+ – 2+ – – 2+ Ni 2+ +2e – → Ni PbSO 4 +2e → Pb+SO – –0.23 2– 4 –0.35 Cd 2+ +2e – → Cd –0.40 Fe +2e → Fe –0.44 Cr 3+ +3e – → Cr –0.73 2+ – Zn +2e → Zn 2+ – 2H 2 O+2e → H 2 +2OH – M n +2e → M n 2+ Al 3+ – + 3e → Al – –0.76 – –0.83 –1.18 –1.66 M g 2+ +2e – → M g –2.37 Na +e → Na –2.71 Ca 2+ +2e – → Ca –2.76 K +e → K –2.92 Li + +e – → Li –3.05 + + – – NCDPI Reference Tables for Chemistry (adopted 2000) Increasing strength as reducing agent Standard Reduction Table 25°C vii viii Rf (261) La 138. 89 Ac Actinium 227.0 58 Ce 88 87 232.0 Thorium 90 Th 140.1 Cerium 104 Lanthanum Ba Barium 137. Cs Cesium 132. Ra Fr Francium Radium 226.0 (223) 76 75 74 73 72 57 56 55 Sg (263) 61 Pm 60 Nd 59 Pr 231.0 Protactnium 91 Pa 140.9 238.0 Uranium 92 U 144.2 237.0 Neptunium 93 Np (145) Neodymium Promethium Seaborgium Db Dubnium (262) Rutherfordium Praseodymium 106 105 108 (244) Plutonium 94 Pu 150.4 Samarium 62 Sm (243) Americium 95 Am 152.0 Europium 63 Eu Hs Bh Bohrium Hassium (265) (262) 107 Os Re W Ta Hf Hafnium Tantalum Tungsten Rhenium Osmium 190.2 186. 183.8 181.0 178. 78 (247) Curium 96 Cm 157.3 (247) Berkelium 97 Bk 158.9 Terbium 99 Es (252) 98 Cf (251) 164.9 Californium Einsteinium 162.5 Holmium 67 Ho (277) Uub Ununbium 112 (257) Fermium 100 Fm 167.3 Erbium 68 Er Tl Hg Mercury Thallium 204.38 200.59 Dysprosium 66 Dy 65 Tb 64 Gd Gadolinium (272) Uuu (269) Uun 111 (266) Mt 110 Meitnerium Ununnilium Unununium 109 Pt Ir Iridium Platinum 195.08 192.22 77 Au Gold 196.96 (258) Mendelevium 101 Md 168.9 Thulium 69 Tm Pb Lead 207.2 82 81 80 79 101.1 (98) 52 84 Xe Xenon 131.3 86 I Iodine 126.9 85 (259) Nobelium 102 No 173.0 Ytterbium 70 Yb (262) Lawrencium 103 Lr 175.0 71 Lu Lutetium Rn Radon (222) 54 Kr Krypton 83.80 53 At Po Bi Bismuth Polonium Astatine (210) (209) 208.98 83 Te Sb Antimony Tellurium 127.6 121.8 Sn Tin 118.7 In Indium 114.8 Cd Cadmium 112.4 95.94 Zr Pd Rh Rhodium Palladium 106.4 102.9 Ag Silver 107.9 Ru Tc Mo 51 50 49 48 47 46 45 44 Molybdenum Technetium Ruthenium 43 42 41 72.61 91.22 40 Ge Nb Niobium 92.91 39 As Arsenic 74.92 Germanium Ga Gallium 69.72 Zn Zinc 65.39 Cu Copper 63.55 Ni Nickel 58.69 Co Cobalt 58.93 Fe Iron 55.85 Zirconium 38 54.94 Mn Manganese Y Sr Rb Rubidium Strontium Yttrium 88.91 87.62 85.47 37 Cr V Ti Sc Ca K Potassium Calcium Scandium Titanium Vanadium Chromium 52.00 50.94 47.88 44.96 40.08 39.10 32 31 30 29 28 27 26 Br Se Selenium Bromine 79.90 78.96 36 35 25 24 23 22 21 34 20 19 26.98 12 2B 11 1B 10 8B 9 8B 8 8B 30.97 33 24.31 7 7B P Al Mg 6 6B Ar Argon 39.95 Cl Chlorine 35.45 S Sulfur 32.07 Phosphorus Si Silicon 28.09 Aluminum Magnesium Na Sodium 22.99 5 5B 18 17 16 15 14 13 12 11 4 4B Ne Neon 20.18 F Fluorine 19.00 O Oxygen 16.00 N Nitrogen 14.01 C Carbon 12.01 B Boron 10.81 3 3B 10 9 8 7 6 5 4 Be Li Lithium Beryllium 9.012 6.941 3 17 7A 16 6A 15 5A 14 4A 13 3A 2 2A He Helium 4.003 18 8A H Hydrogen 1.008 PERIODIC TABLE OF THE ELEMENTS 2 1 1A 1 Stock No. 7952 NCDPI Reference Tables for Chemistry (adopted 2000)