Molecular Weight Determination From Freezing Point Depression
advertisement

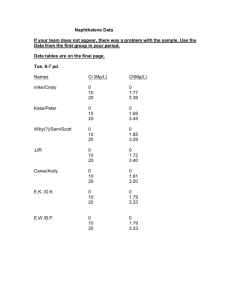
Experiment 5: Molecular Weight Determination From Freezing Point Depression PURPOSE To become familiar with colligative properties and to use them to determine the molar mass of a substance APPARATUS AND CHEMICALS: -Naphthalene -Sulfur or unknown solid -Wire gauze -Thermometer -Large test tube -Wire stirrer -Bunsen burner and hose -Clamp -Ring and ring stand -600-mL beaker -2-hole rubber stopperwith slit -Towel -Wide-mouth glass bottle -Weighing paper THEORY Solutions are homogeneous mixtures that contain two or more substances. The major component is called the solvent, and the minor component is called the solute. Since the solution is primarily composed of solvent, physical properties of a solution resemble those of the solvenL Some of these physical properties, called colligative properties, are independent of the nature of the solute and depend only upon the solute concentration. The colligative properties include vapor-pressure lowering, boiling point elevation, freezing-point lowering, and osmotic pressure. The vapor pressure is just the escaping tendency of the solvent molecules. When the vapor pressure of a solvent is equal to atmospheric pressure, the solvent boils. At this temperature the gaseous and liquid states of the solvent are in dynamic equilibrium, and the rate of molecules going from the liquid to the gaseous state is equal to the rate of molecules going from the gaseous state to the liquid state. it has been found experimentally that the dissolution of a non-volatile solute (one with very low vapor pressure) in a solvent lowers the vapor pressure of the solvent, which in tum raises the boiling point and lowers the freezing poinL This is shown graphically by the phase diagram given in Figure 12.1. You are probably familiar with some common uses of these effects: Anti-freeze is used to lower the freezing point and raise the boiling point of coolant (water) in an automobile radiator; and salt is used to melt ice. These effects are expressed quantitatively by the colligative-property law, which states that the freezing point and boiling point of a solution differ from those of the pure solvent by amounts that are directly proportion al to the molal concentration of the solute. This relationship is expressed by Equation 1 for the freezing-point lowering and boiling-point elevation: Where ∆T is the freezing-point lowering or boiling-point elevation, K is a constant that is specific for each solvent, and m is the molality of the solution (number of moles solute per /1000g solvent). Some representative constants, boiling points, and freezing points are given in Table 12.1. For naphthalene, the solvent used in this experiment, the molal freezing-point depression constant (Kf) has a value of 6.90°C/m. 1 TAB LE 12.1 Molal Freezing-Point and Boiling-Point Constants Solvent Freezing point (°C) Kfp(°C/m) Boiling point (°C) Kbp(°C/m) CH3COOH (acetic acid) 16.6 3.90 118.1 2.93 C6H6 (benzene) 5.4 5.12 80.2 2.53 CHCl3 (chloroform) -63.5 4.68 61.3 3.63 C2H5OH (ethyl alcohol) -114.1 - 78.4 1.22 0.51 H20 (water) 0.0 1.86 100.0 C10H8(naphthalene) 80.6 6.9 218 - C6H12 (cyclohexane) 6.6 20.4 80.17 2.79 EXAMPLE 1: What would be the freezing point of a solution containing 19.5g of biphenyl (C12H10) dissolved in 100g of naphthalene if the normal freezing point of naphthalene is 80.6 °C? Moles C12H10 = 19.5 g 0.127mol 154 g / mol molesC12 H10 0.127mol (1000 g ) 1.27m 1000 gnaphtalen e 100 g ∆T = (6.91 ˚C/m) ×(1.27m) = 8.8 ˚C Since the freezing point is lowered, the observed freezing point of the solution will be 80.6˚C - 8.8˚C = 71.8 ˚C Since the molal freezing-point-depression constant is known, it is possible to obtain the molar mass of a solute by measuring the freezing point of a solution and the weight of both the solute and solvent. 2 EXAMPLE 2: What is the molar mass of urea if the freezing point of a solution containing 15g of urea in 100g of naphthalene is 63.3˚C? SOLUTION: The freezing point of pure naphthalene is 80.6˚C. Therefore, the freezing-point lowering (∆T) is: ∆T = 80.6˚C - 63.3˚C = 17.3˚C From equation 1 above, 17.3˚C = Kfp . m m = 17.3˚C / Kfp Kfp for naphthalene is 6.9˚C/m. Therefore, molality of this solution is; m 17.3 2.5m 6.9 Remember that molality is the number of males of solute per 1000 g of solvent. In our solution there are 15 g urea in 100 g of naphthalene, or 150 g of urea in 1000 g of naphthalene. Thus 150 g = 2.5 mol 1 mol = 60 g The molar mass of urea is, therefore, 60 g/mol. In this experiment you will determine the molar mass of either sulfur or an unknown. You will do this by determining the freezing-point depression of a naphthalene solution having a known concentration of either sulfur or your unknown. The freezing temperature is difficult to ascertain by direct visual observation because of a phenomenon called super cooling and also because solidification of solutions usually occurs over a broad temperature range. Temperaturetime graphs, called cooling curves, reveal freezing temperatures rather clearly. Therefore, you will study the rate at which liquid naphthalene and its solutions cool and will construct a cooling curve similar to the one shown in Figure 12.2. 3 You will construct cooling curves for both the pure solvent and the solution. Figure 12.2 shows how the freezing point of a solution must be determined by extrapolation of the cooling curve. Extrapolation is necessary because as the solution freezes the solid that is formed is essentially pure solvent and the remaining solution becomes more and more concentrated. Thus its freezing point lowers continuously. Clearly, super cooling produces an ambiguity in the freezing point and should be minimized. Stirring the solution helps to minimize super cooling. REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. Distinguish between solute and solvent. 2. List three colligative properties and suggest a rationale for the choice of the word colligative to describe these properties. 3. Distinguish between volatile and non volatile substances 4. What effect does the presence of a nonvolatile solute have upon (a) the vapor pressure of a solution, (b)the freezing point, and (c) the boiling point? 5. What is the molality of a solution that contains 1.5 g urea (molar mass = 60 amu) in 200 g of benzene? 6. What is supercooling? How can it be minimized? 7. Calculate the freezing point of a solution containing 6.50 g of benzene in 160 g of chloroform. 8. A solution containing 1.00 g of an unknown substance in 12.5 g of naphthalene was found to freeze at 75.4°C. What is the molar mass of the unknown substance? 9. How many grams of NaN03 would you add to 250 g of H20 in order to prepare a solution that is 0.200 molal in NaN03? 10. Define molality, m and molarity, M. PROCEDURE A. Cooling Curve for Pure Naphthalene 1. Weigh a large test tube to the nearest 0.01 g. Add about 15 g of naphthalene and weigh again. The difference in weight is the weight of naphthalene. 2. Assemble the apparatus as shown in Figure 12.3; be certain to use a split two-hole rubber stopper. Carefully insert the thermometer into the hole that has been slit. Bend the stirrer so that the loop encircles the thermometer. 3. Fill your 600-mL beaker nearly full of water and heat it to about 850C. Clamp the test tube in the water bath as shown in Figure 12.3. 4 4. When most of the naphthalene has melted, insert the stopper containing the thermometer and stirrer into the test tube; make certain that the thermometer is not resting on the bottom of or touching the sides of the test tube. When all of the naphthalene has melted, stop heating, remove the beaker of water, and dry the outside of the test tube with a doth towel. 5. Record temperature reading s every 30 s while you are stirring. When the freezing point is reached, crystals will start to form, and the temperature will remain constant. Shortly after this, the naphthalene will solidify to the point where you can no longer stir it. (Your lab instructor will direct you to perform either procedure B or C.) B. Determination of the Molar Mass of Sulfur 1. Using weighing paper, weigh to the nearest 0.01 g about 1.2 to 1.5 g of sulfur. CLEAN UP ANY SULFUR SPILLS IN THE BALANCE. 2. Replace the test tube in the water bath and heat until all the naphthalene has melted. Gently remove the stopper, making sure that no naphthalene is lost, and add the sulfur to the test tube. 3. Replace the stopper and stir gently until all the sulfur has dissolved. 4. Remove the water bath, dry the test tube with a towel. Record the temperature every 30 s until all the naphthalene has solidified. Clean-up !!!.. To clean out the test tube at the end of the experiment, heat the test tube in a water bath until the naphthalene just melts. Care should be taken not to heat the thermometer beyand Us temperature range. Be careful, because naphthalene is flammable. Remove the stopper and pour the molten naphthalene on a crumpled wad of paper. When the naphthalene has solidified, throw both the paper and solid naphthalene into a waste receptacle. DO NOT POUR LIQUID NAPHTHALENE INTO THE SINK! 5 C. Determination of the Molar Mass of Unknown 1. Place the test tube in the water bath and heat until all the naphthalene has melted. 2. Using weighing paper, weigh about 2 g of your unknown to the nearest 0.01 g. Gently remove the stopper from the test tube, making sure that no naphthalene is lost, and add your unknown to the test tube. 3. Replace the stopper and stir gently until all the unknown has dissolved. Remove the water bath, dry the test tube with a towel. 4. Record the temperature every 30 s until all the naphthalene has solidified. Clean up as described in Part B above. QUESTIONS 1. What are the major sources of error in this experiment? 2. Arrange the following aqueous solutions in order of increasing freezing points (lowest to highest temperature): 0.10 m glucose, 0.10 m BaCl2 0.20 m NaCl, and 0.20 m Na2S04. 3. What mass of NaCl is dissolved in 150 g of water in a 0.050 m solution? 4. Suppose your thermometer consistently read a temperature 1.2oC lower than the correct temperature throughout the experiment. How would this have affected the molar mass you found? 5. If the freezing point of the solution had been incorrectly read 0.30°C lower than the true freezing point, would the calculated molar mass of the solute be too high or too low? Explain your answer. 7. A solution of 2.00 g of para-dichlorobenzene in 50.0 g of cyclohexane freezes at 1.05°C. What is the molar mass of this substance? 6 DATA SHEET Molecular Weight Determination From Freezing Point Depression Student's Name : Date: Laboratory Section/Group No : Assistant's Name and Signature: 1. Weight of naphthalene : 2. Weight of naphthalene : 3. Weight of sulfur or unknown : 4. Weight of sulfur or unknown : 5. Freezing point of pure naphthalene, from cooling curve …………….. °C 6. Freezing point of solution of sulfur or unknown in naphthalene ………°C 7. Molality of sulfur or unknown : 8. Molar mass of sulfur or unknown : 7 ∆T = ….°C COOLING CURVE Pure naphtalene Temperature (°C) Naphtalene + sulfur or unknown Time (s) Temperature (°C) 8 Time (s) 9