Name
CHAPTER 6
Class
Date
The Structure of Matter
SECTION
1 Compounds and Molecules
KEY IDEAS
As you read this section, keep these questions in mind:
• What holds compounds and substances together?
• What determines the properties of a substance?
What Are Chemical Bonds?
Table salt and sugar look similar, but they have very
different tastes. Their similarities and differences are
partly due to the way their atoms or ions are joined.
Recall that compounds are substances that are made
of two or more elements. Chemical bonds are forces
that hold atoms or ions together in a compound. The
chemical bonds can break and re-form during chemical
changes.
READING TOOLBOX
Define As you read this
section, write down any
science terms you do not
understand. Find the
definitions of these terms
in earlier chapters and write
them in the sidebar.
READING CHECK
1. Define What is a
chemical bond?
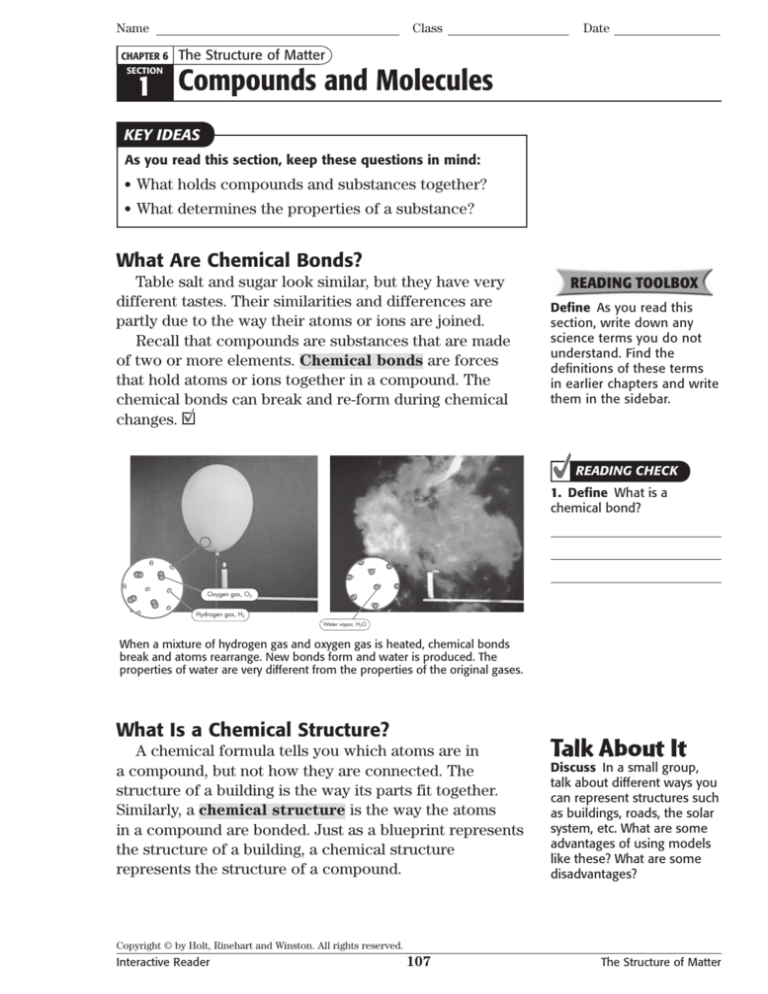
Oxygen gas, O2
Hydrogen gas, H2
Water vapor, H2O
When a mixture of hydrogen gas and oxygen gas is heated, chemical bonds
break and atoms rearrange. New bonds form and water is produced. The
properties of water are very different from the properties of the original gases.
What Is a Chemical Structure?
A chemical formula tells you which atoms are in
a compound, but not how they are connected. The
structure of a building is the way its parts fit together.
Similarly, a chemical structure is the way the atoms
in a compound are bonded. Just as a blueprint represents
the structure of a building, a chemical structure
represents the structure of a compound.
KXcb8Yflk@k
Discuss In a small group,
talk about different ways you
can represent structures such
as buildings, roads, the solar
system, etc. What are some
advantages of using models
like these? What are some
disadvantages?
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
107
The Structure of Matter
Name
SECTION 1
Class
Date
Compounds and Molecules continued
How Are Chemical Structures Represented?
Scientists use different chemical models to show
different characteristics of compounds. Some models
show the position of atoms in the molecule. Other
models show the relative sizes of each atom in the
compound. Ball-and-stick and space-filling models are
two of the most commonly used chemical models.
Space-filling model
Ball-and-stick model
EHHDBG@<EHL>K
2. Compare What does the
space-filling model of a water
molecule tell you about the
relative size of the atoms?
O
H
104.45º
95.8 pm
Oxygen atom
H
Hydrogen atoms
The ball-and-stick model shows angles between bonds more clearly than the
space-filling model does. However, the space-filling model shows the relative
sizes of atoms more clearly than the ball-and-stick model does.
BALL-AND-STICK MODELS
READING CHECK
3. Explain How do structural formulas differ from
ball-and-stick models?
Scientists use two terms to describe the relative
positions of atoms in a compound. Bond length is the
distance between the nuclei of two bonded atoms. Bond
angle is the angle formed by two bonds connected to the
same atom. A molecule must have three or more atoms to
have a bond angle.
A ball-and-stick model uses balls to represent atoms
and sticks to represent chemical bonds. This type of
model is useful because it shows clearly the bonds and
angles between atoms.
A ball-and-stick model shows you how the atoms or
ions are arranged in a compound. Look again at the balland-stick model for water above. Hydrogen and oxygen
atoms bond to form a molecule that appears bent. The
molecule looks more like a boomerang than a straight
line.
You can also use structural formulas to show the structure of a compound. Structural formulas are similar to
ball-and-stick models. However, in a structural formula,
chemical symbols represent the atoms.
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
108
The Structure of Matter
Name
SECTION 1
Class
Date
Compounds and Molecules continued
Structural formula
O
H
H
SPACE-FILLING MODELS
A space-filling model shows the relative amount of
space each atom takes up. In other words, a space-filling
model can show relative sizes of atoms. However, unlike
ball-and-stick or structural models, space-filling models
do not show bond lengths clearly.
REPRESENTING BONDS
Bonds are not really like sticks in a ball-and-stick
model. Although bonds hold atoms tightly together, most
bonds can bend, stretch, and rotate without breaking.
Thus, you can think of bonds as flexible springs rather
than rigid sticks.
READING CHECK
4. Identify What is one
disadvantage of space-filling
models?
EHHDBG@<EHL>K
5. Explain Why are bonds
more like springs than like
sticks?
Scientists generally use a straight, solid line to show a bond between
two atoms. However, bonds are actually flexible like springs.
How Does Chemical Structure Affect
Chemical Properties?
The chemical structure of a compound determines the
compound’s properties. Some substances, such as quartz,
are made up of large networks of bonded atoms. Other
substances, such as table salt, are made up of networks
of positive and negative ions. Some substances, such as
water or sugar, are made of separate molecules.
The compounds that make up different substances
join together in different ways when the substances are
solids. The atoms in some substances, such as quartz,
are connected strongly. These compounds tend to form
strong or hard solids.
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
109
The Structure of Matter
Name
SECTION 1
Class
Date
Compounds and Molecules continued
EHHDBG@<EHL>K
6. Identify How does the
chemical structure of quartz
make the substance rigid?
The bonds that hold oxygen and silicon atoms together are very strong.
The strength of the bonds between atoms makes quartz hard and rigid.
8g^i^XVaI]^c`^c\
The molecules of substances such as sugar are bonded
together more weakly. For example, atoms within each
molecule of sugar are strongly attracted to each other,
but individual molecules are not. Thus, sugar and similar
substances tend to be softer and melt more easily.
7. Explain Why do substances such as sugar dissolve
more easily than substances
such as quartz?
/XYGENATOM
(YDROGENATOM
#ARBONATOM
Each grain of sugar is made up of many sugar molecules, C12 H22 O11.
READING CHECK
Substances made of ions, such as sodium chloride,
NaCl, are joined together by attractions between ions.
The ions form a regular repeating network held together
by strong bonds between ions with opposite charges.
The strong attractions between ions give ionic
compounds high melting and boiling points.
8. Explain Why do ionic
compounds tend to have
high melting and boiling
points?
Chloride ion, Cl–
Sodium ion, Na+
Each grain of table salt, or NaCl, is made of a tightly packed network of ions.
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
110
The Structure of Matter
Name
SECTION 1
Class
Date
Compounds and Molecules continued
How Are Attractions Between Particles
Related to State?
At room temperature, attractions between particles in
a solid are stronger than those between particles of a
liquid. Therefore, sugar molecules attract one another
more strongly than water molecules do. Similarly,
particles in a liquid attract one another more strongly
than particles in a gas do.
What Are Hydrogen Bonds?
The chemical structures of water and dihydrogen
sulfide are similar. Why, then, does water have much
higher melting and boiling points than dihydrogen sulfide
does?
Water molecules are pulled together by attractions
called hydrogen bonds. In a hydrogen bond, oxygen
atoms and hydrogen atoms of different water molecules
are attracted to one another. Although hydrogen bonds
can pull water molecules together, hydrogen bonds are
not as strong as chemical bonds.
Strong bonds
within each
water molecule
READING CHECK
9. Compare How does the
strength of attraction among
particles differ in solids and
liquids?
READING CHECK
10. Compare Which is
stronger—a hydrogen bond
or a chemical bond?
Weaker attractions
between water
molecules
Water is a liquid at room temperature instead of a gas because
hydrogen bonds hold water molecules together.
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
111
The Structure of Matter
Name
Class
Date
Section 1 Review
SECTION VOCABULARY
bond angle the angle formed by two bonds to
the same atom
bond length the distance between two bonded
atoms at their minimum potential energy;
the average distance between the nuclei of
two bonded atoms
chemical bond the attractive force that holds
atoms or ions together
chemical structure the arrangement of the
atoms in a molecule
1. Explain Why do scientists use different types of models to represent compounds?
2. Identify Which type of chemical model shows the bond angle and bond length
between atoms in the compound? How does this type of model represent a
compound?
3. Interpret Draw a ball-and-stick model of a boron trifluoride molecule. In this
molecule, three fluorine atoms are attached to a boron atom. Each F-B-F bond
angle is 120°, and all B-F bonds are the same length.
4. Predict Which molecules are more strongly attracted to one another—C3H8O
molecules that make up liquid rubbing alcohol or CH4 molecules that make up
methane gas? Explain your answer.
5. Apply Concepts What can you infer about the attraction between particles in a
substance with a low melting point?
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
112
The Structure of Matter









