P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
Annu. Rev. Cell Dev. Biol. 1999. 15:33–62
c 1999 by Annual Reviews. All rights reserved
Copyright BLUE-LIGHT PHOTORECEPTORS
IN HIGHER PLANTS
?
Winslow R. Briggs
Department of Plant Biology, Carnegie Institution of Washington, Stanford, California
94305; e-mail: briggs@andrew2.stanford.edu
Eva Huala
Department of Genetics, Stanford University School of Medicine, Stanford, California
94305; e-mail: huala@genome.stanford.edu
Key Words cryptochrome, flavoproteins, LOV domain, PAS domain, phototropin
■ Abstract In the past few years great progress has been made in identifying and
characterizing plant photoreceptors active in the blue/UV-A regions of the spectrum.
These photoreceptors include cryptochrome 1 and cryptochrome 2, which are similar in structure and chromophore composition to the prokaryotic DNA photolyases.
However, they have a C-terminal extension that is not present in photolyases and lack
photolyase activity. They are involved in regulation of cell elongation and in many
other processes, including interfacing with circadian rhythms and activating gene transcription. Animal cryptochromes that play a photoreceptor role in circadian rhythms
have also been characterized. Phototropin, the protein product of the NPH1 gene in
Arabidopsis, likely serves as the photoreceptor for phototropism and appears to have
no other role. A plasma membrane protein, it serves as photoreceptor, kinase, and
substrate for light-activated phosphorylation. The carotenoid zeaxanthin may serve as
the chromophore for a photoreceptor involved in blue-light-activated stomatal opening.
The properties of these photoreceptors and some of the downstream events they are
known to activate are discussed.
CONTENTS
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
The Cryptochrome Family of Photoreceptors . . . . . . . . . . . . . . . . . . . . . . . . . 35
The Cryptochrome 1 Gene and Protein . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Components of Signal Transduction Downstream
from Cryptochrome 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Processes Mediated by Cryptochrome 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The Cryptochrome 2 Gene and Protein . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Processes Mediated by Cryptochrome 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Cryptochromes in Other Photosynthetic Organisms . . . . . . . . . . . . . . . . . . . . . . .
1081-0706/99/1115-0033$08.00
37
39
41
41
42
33
P1: FHN/fgi
P2: FHN
September 20, 1999
34
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
Cryptochromes and Plant Circadian Rhythms . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
Cryptochromes in Animals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
Phototropin (nph1) and Related Proteins . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
Phototropin (nph1) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
The LOV Domains . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
Components of Signal Transduction Downstream from Phototropin . . . . . . . . . . . 48
Arabidopsis NPL1 and Adiantum PHY3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
A Blue-Light Photoreceptor for Stomata . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Co-action Between Photoreceptor Systems . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
The Phototropism Pathway . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
Cryptochrome Pathways . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Action Spectra and Nomenclature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
The Next Steps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
?
INTRODUCTION
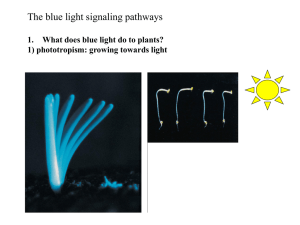
Phototropism is the curvature of growing plant organs toward or away from a
source of unilateral light. It is a classic plant response to blue light and has been
investigated for more than a century. Charles Darwin (1881) first showed that
red light did not induce this curvature response, and it was Julius von Sachs in
1883 (see Sachs 1887) who measured the first crude action spectrum with colored
glass and colored solutions, demonstrating that wavelengths in the blue region of
the spectrum were among those that did induce a response. These phototropism
studies were carried out more than half a century before the first demonstration that
longer wavelengths of light could affect plant development. Flint (1934) and Flint
& McAlister (1935, 1937) were the first to report experiments showing that red
light promoted lettuce seed germination and that far-red light inhibited it. Flint
& McAlister’s work formed the genesis of an entire field of research that was
subsequently designated photomorphogenesis. Their work has led to our present
knowledge of the phytochrome family of photoreceptors and the physiological,
biochemical, and molecular consequences of conversion of the red-absorbing form,
Pr, to the far-red-absorbing form, Pfr (Batschauer 1998, Chamovitz & Deng 1996,
Fankhauser & Chory 1997, Quail et al 1995, Quail 1997, Whitelam & Devlin 1998).
Butler et al (1959) used biochemical and spectral techniques to demonstrate that
phytochrome was a chromoprotein. By contrast, research on the nature of bluelight photoreceptors lagged behind. It was only in 1993 that Ahmad & Cashmore
reported the first sequence for cryptochrome 1 (cry1), a chromoprotein that serves
as a blue-light photoreceptor. [We use here the notation style recommended for
the phytochromes (Quail et al 1994): e.g. cry1 for the holoprotein, CRY1 for the
apoprotein, CRY1 for the wild-type gene, and cry1 for the mutated gene.]
Although there was an enormous literature on the many physiological, biochemical, and molecular responses of plants (as well as fungi and algae) to blue light
prior to 1993 (for reviews, see Briggs & Iino 1983; Jenkins et al 1995; Kaufman
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
35
1993; Senger 1980, 1984, 1987; Senger & Briggs 1981; Short & Briggs 1994),
there was anything but agreement as to what might be the nature of the blue-lightabsorbing chromophore. Candidate chromophores were carotenoids (Quiñones &
Zeiger 1994, Quiñones et al 1996, Shropshire 1980, Wald & du Buy 1936), flavins
(Galston 1949, 1950), pterins (Galland & Senger 1988), and even retinal (Lorenzi
et al 1994). Briggs et al (1957) rejected Galston’s proposal for flavins when they
found that the hypothesized differential flavoprotein-mediated destruction of the
growth hormone auxin across a unilaterally irradiated plant organ did not occur. (However, involvement of flavins in other mechanisms was not considered.)
Palmer et al (1996) later rejected carotenoids because maize coleoptiles devoid
of detectable carotenoids responded to unilateral blue light with normal phototropic curvature (and blue-light-activated phosphorylation, see below). Action
spectroscopy was of no particular help in identifying the chromophore (Briggs &
Iino 1983). What later came to be called the cryptochrome-type action spectrum-a
single broad action band in the UV-A and a band with fine structure in the blue–was
used by both the carotenoid and flavin camps to support their candidates. This
was because it showed spectral properties that were characteristic of each putative
chromophore (fine structure in the blue, typical of carotenoids, and a broad peak
in the UV-A, typical of flavins and flavoproteins). Biochemistry failed to resolve
the issue, and a genetic approach was clearly in order.
Since the seminal paper by Ahmad & Cashmore (1993), research on bluelight receptors has rapidly accelerated, as indicated by the spate of recent reviews
(Ahmad & Cashmore 1996, Batschauer 1998, Cashmore 1997, Cashmore et al
1999, Chamovitz & Deng 1996, Fankhauser & Chory 1997, Jenkins et al 1995,
Jenkins 1997, Khurana et al 1998, Lin & Cashmore 1996, Liscum & Hangarter
1994, Ninnemann 1997, Whitelam & Devlin 1998). In the present review, we
first summarize research on three recently characterized blue-light photoreceptors
in plants, cryptochrome 1 (cry1), cryptochrome 2 (cry2), and phototropin (nph1,
the photoreceptor for phototropism), and review progress in the identification of
a fourth blue-light photoreceptor, which mediates light-activated stomatal opening. We discuss studies in which mutants have implicated specific photoreceptors
in various downstream signaling steps and processes and briefly treat the interaction of pathways activated by different photoreceptors. We also review studies
on organisms other than plants where sequence comparisons indicate evolutionary
conservation of specific domains and include the recent work on cryptochromes
and their possible function in animals.
?
THE CRYPTOCHROME FAMILY OF PHOTORECEPTORS
It was Gressell (1977) who coined the term cryptochrome to refer to the then
unidentified photoreceptor(s) with the cryptochrome-type action spectrum described above. The tacit assumption was that the cryptochrome family of photoreceptors would be related proteins, as are the phytochromes, and would share a
P1: FHN/fgi
P2: FHN
September 20, 1999
36
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
common chromophore. Thus Lin et al (1995a) referred to the putative photoreceptor protein encoded by the HY4 locus, described by Ahmad & Cashmore (1993),
as cryptochrome 1 or cry1. (Earlier-described mutant alleles at this locus are still
designated hy4, but more recently described alleles are designated cry1.) The discovery of the gene for a closely related protein, cryptochrome 2 or cry2, and the
preliminary characterization of its protein product (Hoffman et al 1996, Lin et al
1996b) solidified the cryptochrome nomenclature in the literature. We return to
the question of nomenclature for blue-light photoreceptors in a subsequent section.
?
The Cryptochrome 1 Gene and Protein
Mutations at the HY4 locus in Arabidopsis fail to show blue-light-induced inhibition of hypocotyl elongation (Koornneef et al 1980). Ahmad & Cashmore (1993)
isolated a T-DNA-tagged mutant, hy4-2, allelic with the original hy4 allele, hy42.23N, from Koornneef’s laboratory, permitting them to clone and sequence the
wild-type HY4 gene. The gene encodes a 681-amino acid protein with sequence
identity near 30% with prokaryotic DNA photolyases over the N-terminal 500
amino acids. Identities are as high as 70% or more over domains involved in
chromophore binding in the photolyases. Identification of mutations in the CRY1
sequences of three other hy4 alleles [plus many more subsequently characterized
(Ahmad et al 1995)] confirmed the identification of the gene.
At its C-terminal end, cry1 has an extension, found in none of the photolyases,
that shows some relatedness to rat smooth muscle tropomyosin. However, this
extension lacks the predicted α-helicity characteristic of tropomyosin, making it
difficult to evaluate the functional relevance of the similarity (Ahmad & Cashmore
1996). Nevertheless, seven of the alleles have mutations in this region (Ahmad &
Cashmore 1993, Ahmad et al 1995), suggesting its importance for cry1 function.
Because the photolyases serve as photoreceptors mediating light-activated repair
of pyrimidine dimers in UV-damaged DNA, Ahmad & Cashmore (1993) proposed
that the hy4 holoprotein was itself a photoreceptor, mediating blue-light-induced
inhibition of hypocotyl elongation.
Photolyase activity was unlikely to account for cry1 action on elongation growth
because the protein lacks a specific tryptophan (W277 in Escherichia coli photolyase) that is conserved in all prokaryotic photolyases and is required for binding
the enzyme to the damaged DNA (Li & Sancar 1990). Indeed, subsequent work
failed to detect cry1 photolyase activity (Lin et al 1995b, Malhotra et al 1995).
Pang & Hays (1991) had already demonstrated photolyase activity in Arabidopsis
both in vivo and in vitro, and Ahmad et al (1997) recently cloned and characterized a single-copy gene, designated PHR1, that encodes a protein similar to animal
type II photolyases. Complementation of an E. coli photolyase mutant with PHR1
and identification of an Arabidopsis mutant lacking photolyase activity and with
a lesion in the PHR1 gene, demonstrated that phr1 was an authentic photolyase.
Surprisingly, the gene showed little sequence similarity, either with the prokaryotic
type I photolyases or with the cryptochromes.
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
37
Photolyases all have flavin adenine dinucleotide (FAD) and either a deazaflavin
or a pterin as the chromophore (Sancar 1994). Cry1 was found to bind the expected
FAD (Lin et al 1995b, Malhotra et al 1995). Thus Galston’s original hypothesis
(1949, 1950) that a flavin could serve as the chromophore in a plant photoreceptor has finally been confirmed. Although flavin-binding domains are in most
cases poorly conserved, there is some conservation within the photolyases. The
flavin-binding domains of photolyases using deazaflavin as a second chromophore
show greater sequence similarity to others using a deazaflavin than those using a
pterin (see Malhotra et al 1992 and references therein) and vice versa. Although
the sequence similarity of the chromophore-binding domain originally indicated
that cry1 might bind a deazaflavin as its second chromophore (Lin et al 1995b),
Malhotra et al (1995) found it to be the pterin methenyltetrahydrofolate (MTHF)
at least for the protein produced by the expression of HY4 in E. coli. If cry1 in
vivo also binds MTHF, the first blue-light photoreceptor characterized would then
have two of the four originally proposed chromophore molecules.
Subsequent studies from the Cashmore laboratory provided evidence for the
hypothesis that cry1 was a true photoreceptor (Ahmad & Cashmore 1993). First,
Arabidopsis itself is something of an oddity in that several responses are elicited
by green light in addition to blue and UV-A light (phototropism: Konjević et al
1989, 1992; hypocotyl inhibition: Young et al 1992). Lin et al (1995b) noted under
redox conditions that might be found in plant cells, cry1 holoprotein formed a stable
flavosemiquinone with significant absorption in the green. Second, overexpression
of cry1 in tobacco gave the seedlings a dramatic increase in the sensitivity for
inhibition of the hypocotyl not just to blue and UV-A but green light as well
(Lin et al 1995a). Examination of several over-expressing lines showed that the
amount of increased hypocotyl inhibition compared with wild-type was closely
correlated with the degree of over-expression. Third, over-expression of cry1 in
Arabidopsis also caused hypersensitivity to all three wavelength regions (Lin et al.
1996a). Together with cry1’s obvious similarities in structure and chromophores
with the DNA photolyases, known to be photoreceptors, these observations leave
little doubt that cry1 functions as a photoreceptor.
Cry1 appears to be a soluble protein (Lin et al 1996a), although it remains
possible that a fraction may be membrane associated (Ahmad et al 1998b). It is
expressed both in dark-grown Arabidopsis seedlings and in all organs of mature
light-grown plants (Ahmad & Cashmore 1993). Protein levels of cry1 in Arabidopsis are unaffected by white light treatment (Lin et al 1996a), and in this sense, the
Arabidopsis chromoprotein is analogous to phyB, which is light stable (see Quail
1991).
?
Components of Signal Transduction Downstream
from Cryptochrome 1
To date, nothing is known about the earliest events following cry1 photoexcitation.
Based on analogy with the photolyases, it seems likely that signal transduction may
P1: FHN/fgi
P2: FHN
September 20, 1999
38
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
be initiated by an electron transfer reaction (see discussions in Ahmad & Cashmore
1997, Jenkins et al 1995, Malhotra et al 1995). Indeed, cry1 contains the tryptophan
corresponding to W306 in E. coli. In the prokaryotic photolyases, this tryptophan
donates an electron to photoexcited FADH to generate FADH− as an early event
in the repair mechanism (see Sancar 1994). If such electron transfer is involved
with the plant photoreceptor, cry1 is using a signal transduction mechanism that
differs from other known mechanisms such as protein conformational change,
phosphorylation, changes in protein-protein interaction, and G-protein activation
(Malhotra et al 1995). Given the complex photochemical properties of flavins
(and pterins), the proposal is reasonable. However, there are still no hints as to a
possible cryptochrome reaction partner in the putative redox reaction.
Elements further down the signal-transduction pathway have been identified,
however. A large number of Arabidopsis mutants have been described that show a
light-grown phenotype when grown in darkness. These include the cop (constitutive photomorphogenesis) and det (de-etiolated phenotype) mutants. Many of
these mutants were subsequently found to be identical to the previously described
fus ( fusca) mutants selected because they showed intense accumulation of anthocyanin pigments in darkness. Mutations at the cop/det/fus loci are all recessive,
and because they show the phenotype of a light-grown plant, the gene products of
these loci are thought to act as repressors of photomorphogenesis (see reviews by
Batschauer 1998, Chamovitz & Deng 1996, Fankhauser & Chory 1997, Whitelam
& Devlin 1998).
Extensive work by Deng and associates has shown that the products of a number
of these genes are required to suppress the developmental pattern normally seen
in light-grown seedlings. Furthermore, they must block those components of
photomorphogenesis that are specifically activated by photoexcitation of phyA,
phyB, and cry1. For example, cop1 mutations are epistatic not just to phytochrome
mutations such as hy1, hy2, and hy3, but also to hy4 (cry1) (Ang & Deng 1994).
Likewise, cop8, cop10, and cop11 (Wei et al 1994) and cop12 ( fus12), cop13
( fus11), cop14 ( fus4), cop15 ( fus5), and det1 ( fus2) (Kwok et al 1996) are all
epistatic to the phytochrome and cry1 mutations. Finally, overexpression of the
COP1 gene inhibits normal photomorphogenesis in the light (McNellis et al 1994),
and overexpression of an N-terminal fragment of COP1 has a dominant-negative
effect on light-mediated seedling development (McNellis et al 1996), consistent
with the hypothesis that COP1 acts as a repressor of photomorphogenesis.
The current model is that COP1, plus probably the DET1 protein and other
proteins, is localized in the nucleus in the dark. These proteins associate with a
large complex designated the COP9 complex, which is constitutively localized in
the nucleus and contains 12 subunits. Some of these subunits have been shown
be related to human counterparts, suggesting that the COP9 complex may play a
general role in eukaryotic development (Chamovitz et al 1996). In the light, COP1
moves out of the nucleus to the cytoplasm, where it binds COP-interacting protein
1 (CIP1) (Matsui et al 1995). The various genes involved in photomorphogenesis,
whether regulated by blue or red light through the cryptochrome or phytochrome
?
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
39
photoreceptors, respectively, are thought to be derepressed when COP1 is absent
from the nucleus (Chamovitz & Deng 1996, Chamovitz et al 1996). Recent studies indicate that phyA, phyB, and cry1 are all involved in a somewhat complex
fashion in triggering the COP1 migration in the light (Osterlund & Deng 1998).
How signals arriving from the different photoreceptors converge on a single regulatory complex and become subsequently repartitioned to mediate different and
sometimes completely unrelated responses remains a puzzle.
?
Processes Mediated by Cryptochrome 1
In addition to regulating hypocotyl elongation, cry1 mediates many other processes. Mutants at the HY4 locus showed decreased cotyledon expansion, increased petiole elongation, increased flower stem elongation, and increased leaf
expansion in light-grown seedlings (Jackson & Jenkins 1995). Interestingly, excision of hy4 cotyledons prior to irradiation eliminated the mutant phenotype, and
the cotyledon response to blue light was similar to the response of cotyledons
excised from wild-type seedlings. The results suggest that the cry1 signal must be
transmitted to the cotyledons from another part of the seedlings (Blum et al 1994).
The hy4 mutants also showed decreased formation of anthocyanins (Ahmad et al
1995, Jackson & Jenkins 1995) and reduced blue-light-induced accumulation of
the mRNAs for enzymes such as chalcone synthase (CHS) (Ahmad et al 1995,
Fuglevand et al 1996, Jackson & Jenkins 1995), chalcone isomerase (CHI), and
dihydroflavonol reductase (DFR) (Jackson & Jenkins 1995), all catalysing steps
early in the phenylpropanoid pathway. At least for CHS, blue-light induction was
independent of phyA and phyB, although both phytochromes have absorption in
the blue region of the spectrum (Batschauer et al 1996). In addition to regulating
the transcription of genes encoding enzymes early in the phenylpropanoid pathway, cry1 is also required for full expression of GAPA, GAPB (genes encoding
glyceraldehyde-3-phosphate dehydrogenases A and B), and rbcS, three nuclear
genes encoding chloroplast proteins (Conley & Shih 1995).
Transgenic Arabidopsis seedlings over-expressing CRY1 showed correspondingly increased steady-state levels of CHS mRNA and increased accumulation of
anthocyanins in response to UV-A, blue, and green light (Lin et al 1996a). These
transformants showed pleiotropic effects on seedling morphology, with shorter
petioles, smaller rosettes, reduced leaf size, and shorter inflorescence stems, in direct contrast to the effects found in hy4 mutants (see above). The over-expressing
transgenics also showed slightly delayed flowering. However, this response could
be a function of the ecotype (Wassilewskija). In Columbia, mutants deficient in
cry1 showed greatly delayed flowering under a daylength extension enriched for
blue light or following night interruptions with blue light (Bagnall et al 1996).
Some time ago blue light was shown to induce a rapid and strong inhibition
of elongation growth in etiolated seedlings (onset of inhibition within minutes or
seconds of the start of irradiation) (Cosgrove 1981, Gaba & Black 1979, Meijer
1968). The inhibition is transient and the growth rate recovers upon return to
P1: FHN/fgi
P2: FHN
September 20, 1999
40
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
darkness. Blue light, even in brief pulses, can also induce a lasting inhibition
of growth, but this inhibition is separable from the rapid inhibition. In etiolated
pea seedlings, the long-lasting inhibition does not begin until many hours after a
blue-light pulse and persists for many more hours (Warpeha & Kaufman 1989),
whereas the rapid and transient response shows a lag of only 2–3 min (Laskowski
& Briggs 1989).
Blue light also activates a dramatic and rapid depolarization of the plasma
membrane in etiolated seedlings (Spalding & Cosgrove 1988). This depolarization precedes the rapid inhibition of growth and shows the same fluence-response
characteristics, suggesting that the depolarization is causally related to the growth
response. Initially this effect was thought to be mediated by light activation of
a H+-ATPase (Spalding & Cosgrove 1992). However, subsequent work demonstrating blue-light activation of an anion channel sensitive to the inhibitor 5-nitro(3-phenylpropylamino)-benzoic acid (NPPB) (Cho & Spalding 1996) called the
earlier interpretation into question. Lewis et al (1997) later showed this activation
to follow a calcium-independent pathway. NPPB also strongly inhibited bluelight-induced accumulation of anthocyanins in Arabidopsis, but curiously failed
to show any effect on blue-light-induced increases in the levels of phenylalanine
ammonia lyase 1 (PAL1), CHS, CHI, or DFR mRNA, or proteins (Noh & Spalding
1998). Hence, blue-light induction of anthocyanin accumulation must involve
more than one blue-light-activated pathway, at least one of which is independent of the transcriptional activation of the appropriate genes, but involves anion
channel activation.
As with the COP/DET/FUS complex, mutants are helping to elucidate which of
the above responses are mediated by the cry1 photoreceptor. Using both intracellular and surface-contact electrodes, Parks et al (1998) found blue-light-induced
depolarization to be greatly reduced (though not eliminated) in the Arabidopsis
cry1 null mutant hy4-2.23N. However, the rapid inhibition of growth was unaffected in the mutant. Only a longer-term growth inhibition was affected by the loss
of cry1 protein. NPPB mimicked the effect of the cry1 mutation: It had no effect on
the rapid inhibition of growth by blue light but blocked the longer-term response. It
is not clear whether the Arabidopsis long-term response is similar to that described
for pea, as the latter has a much longer lag period (Warpeha & Kaufman 1989).
In a closely related study, Wang & Iino (1997) carried out elegant photobiological investigations of a blue-light-induced transient shrinking of protoplasts isolated
from maize coleoptiles grown under continuous red light. A blue-light pulse also
transiently inhibited the elongation of intact coleoptiles, and in both cases the kinetics were not dissimilar to those for the rapid inhibition of stem growth. As with
the depolarization response reported by Cho & Spalding (1996), NPPB completely
inhibited the shrinking response. Wang & Iino (1998) investigated the same phenomenon in protoplasts from red-light-adapted Arabidopsis hypocotyls. As with
the coleoptile protoplasts, NPPB strongly inhibited the light-induced shrinking.
The shrinking response was absent in the hy4-1 mutant, implicating cry1 directly
in the response. Despite the relative rapidity of the response, however, it can not
?
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
41
be directly related to the rapid inhibition of growth because this latter inhibition is
unaffected in hy4 mutants (Parks et al 1998). At present the nature of the photoreceptor(s) mediating the rapid inhibition of growth and the residual depolarization
in cry1 mutants discussed above remains unresolved. It will be of interest to carry
out studies similar to those of Parks et al (1998) and Wang & Iino (1997, 1998)
with Arabidopsis cry2 and nph1 mutants (see below).
The Cryptochrome 2 Gene and Protein
?
The CRY2 gene in Arabidopsis encodes a protein of 619 amino acids with extensive similarity to cry1 in the photolyase-like domain (Lin et al 1996b, designated
cry2; Hoffman et al 1996, designated AT-PHH1). Like cry1, cry2 has a C-terminal
extension that is not found in the photolyases. However, the cry2 extension shows
no similarity to that of cry1. Batschauer (1993) cloned a gene from white mustard
(Sinapis alba, SA-PHR1, later designated SA-PHH1), originally thought to be a
plant photolyase. Later work failed to confirm photolyase activity (Malhotra et al
1995), and both SA-PHH1 and cry2, like cry1, lack the conserved tryptophan corresponding to E. coli photolyase amino acid W277 (Malhotra et al 1995, Hoffman
et al 1996). SA-PHH1 is 89% identical to Arabidopsis cry2 at the protein level,
although unlike either Arabidopsis CRY protein, it lacks a C-terminal extension
(Hoffman et al 1996). The chromophore composition of cry2 is currently not fully
resolved, although Lin et al (1996b) report that cry2 binds a flavin.
Arabidopsis cry2 is a soluble protein found throughout the seedling and, unlike cry1, it is strongly down-regulated by blue (but not red) light (Lin et al 1998).
Down-regulation presumably occurs at the protein level because mRNA abundance
is unaffected by blue-light treatment (Lin et al 1998, Ahmad et al 1998a). Regulation is at the level of protein stability and not at the level of protein synthesis
(Ahmad et al 1998a). In this sense, cry2 differs from phyA, in which downregulation by light occurs both at the protein and mRNA levels (see Quail 1991).
Because the down-regulation is unaffected in cry1 mutants, either cry2 itself or
some other blue-light photoreceptor must mediate the response (Lin et al 1998).
To a certain extent cry1 and cry2 have overlapping functions. A number of
chimeric proteins with C-terminal and N-terminal domains swapped between Arabidopsis cry1 and cry2 complemented Arabidopsis hy4-3, a mutant lacking cry1,
both in hypocotyl inhibition and anthocyanin formation (Ahmad et al 1998a).
Chimeric constructs containing cry2 sequences, whether C-terminal or N-terminal,
were unstable in vivo as previously reported for cry2 itself under blue-light treatment (see above). However, cry1 from tobacco, unlike cry1 from Arabidopsis,
is unstable (Ahmad et al 1998a). Hence the stability properties found for the
Arabidopsis cryptochromes are not ubiquitous.
Processes Mediated by Cryptochrome 2
Like cry1, cry2 plays a role in blue-light-induced suppression of stem growth.
Lin et al (1998) examined both seedlings over-expressing CRY2 and cry2 deletion
P1: FHN/fgi
P2: FHN
September 20, 1999
42
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
mutants (see Guo et al 1998) for blue-light inhibition of hypocotyl elongation and
stimulation of cotyledon opening. For the deletion mutants, sensitivity was lost
in the lower but not in the higher fluence-rate range. By contrast, cry1 mutants
showed loss of sensitivity (hypocotyl elongation) only in the high fluence-rate
range. Seedlings over-expressing either cry1 or cry2 showed enhanced growth
inhibition by blue light (Lin et al 1998). Hence at least for the Arabidopsis cryptochromes, as with the phytochromes, there is one pigment (cry2 or phyA) sensitive
to very weak light signals and one sensitive to strong signals (cry1, phyB).
Cry2 also plays a major role in photoperiodic timing. Seedlings carrying cry2
mutations show greatly delayed flowering on long days or in continuous light, and
the cry2 mutation is allelic to the previously described late-flowering mutant fha
(Guo et al 1998). Expression of the CONSTANS (CO) gene, a transcriptional factor
required for long-day promotion of flowering in Arabidopsis, also depends upon
cry2/fha. Seedlings over-expressing CRY2 show increased levels of CO mRNA on
both short and long days, and cry2 mutants show reduced levels, especially on long
days. Additional mutant studies indicated that cry2, acting as a positive regulator
of CO gene expression, mediates the blue-light inhibition of phyB function (Guo
et al 1998). More detailed studies involving both single and double cry mutants
support this hypothesis and also indicate that the roles of cry1 and cry2 are at least
partially redundant (Mockler et al 1999). Mockler et al (1999) also showed that
responses to light quality are elicited only during the first seven days following
germination, with flowering time independent of light quality thereafter. Thus both
the cry1 and cry2 photoreceptors play a role in flowering, a process once thought
to be exclusively controlled by phytochrome in photoperiodically regulated plant
species (see Vince-Prue 1994).
?
Cryptochromes in Other Photosynthetic Organisms
Cryptochromes are likely to be ubiquitous in higher plants and have been identified
in tomato, pea, and rice (see Ahmad & Cashmore 1996). Kanegae & Wada (1998)
identified five genomic clones from the fern Adiantum capillus-veneris with sequence similarity to cry1 and obtained evidence that at least three of these were
expressed. Given the extraordinary complexity of fern photobiological phenomena (Wada & Sugai 1994), the presence of several cryptochromes is not surprising.
Like the Arabidopsis cryptochromes, the predicted fern proteins have C-terminal
extensions, but these sequences bear little relationship to the Arabidopsis extensions (or to each other).
Likewise, Small et al (1995) have isolated a Chlamydomonas cryptochrome
gene, CPH1, encoding a putative CRY protein. It shares 43% identity with SAPHH1 and 49% identity with Arabidopsis cry2. Like both cry1 and cry2, it has
a C-terminal extension. This extension shares little similarity with either of the
Arabidopsis CRY sequences, and Small et al propose that the extension may provide the specificity to interact with the next component in the signal transduction
pathway. It seems reasonable that these downstream components are different for
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
43
the individual cryptochromes. As with the higher-plant cryptochromes, neither
the Chlamydomonas nor the Adiantum CRY proteins have the tryptophan corresponding to W277 in E. coli photolyase.
Cryptochromes and Plant Circadian Rhythms
Cry1 has recently been shown to play a role in perceiving light signals that affect
the oscillator driving circadian rhythms. The mRNA for catalase 3 (CAT3) in
Arabidopsis shows circadian oscillations that dampen to a high steady-state mRNA
level in darkness. In the Arabidopsis hy4-2.23N mutant, which lacks a functional
cry1 protein (Ahmad & Cashmore 1993), no damping was observed over 96 h of
continuous darkness (Zhong et al 1997). Hence cry1 is in some way required for
the damping. In another study, Somers et al (1998) crossed various Arabidopsis
photoreceptor mutants (phyA, phyB, cry1, and cry2) with transgenic plants carrying
the firefly luciferase gene under the control of the Arabidopsis CAB2 promoter.
This construct is highly responsive to the circadian clock (Millar et al 1992).
Using plants carrying the construct, Millar et al demonstrated that a cry1 mutant
showed significant lengthening of the free-running period for CAB2 expression
over wild-type. Somers et al (1998) determined free-running period length under
continuous red or blue light at various fluence rates. Over-expression of CRY1
significantly shortened the free-running period at high fluences of blue or white
light, whereas loss of cry1 resulted in period lengthening over both high and low
fluence-rate ranges. From these studies, Somers et al (1998) concluded that phyB is
the predominant high-intensity red-light photoreceptor, phyA is the low-intensity
red-light photoreceptor, and that both cry1 and phyA perceive and transmit bluelight signals to the clock. The small effects of cry2 are thought to be indirect.
?
Cryptochromes in Animals
In the last four years, interest in CRY genes and proteins has spread well beyond
the realm of plant research with the isolation of CRY genes from humans, mice,
and Drosophila. The human genes hCRY1 and hCRY2 were first isolated as potential photolyase genes [as with SA-PHH1, the cryptochrome from white mustard
(Batschauer 1993)] in an effort to address the ongoing controversy over whether
humans have photolyases (Adams et al 1995, Hsu et al 1996, Todo et al 1996, van
der Spek et al 1996). Like the plant cry1, the human cry proteins carry two chromophores: FAD and a pterin (Hsu et al 1996). The HCRY1 and HCRY2 apoproteins are 73% identical at the amino acid level. As with the plant cryptochromes,
they diverge completely in their C-terminal extensions, which are about 80 amino
acids long.
It was later shown that the human cry1 and cry2 proteins exhibit no photolyase
activity, although unlike the cry proteins discussed above, they appear to have both
of the essential tryptophan residues (corresponding to the E. coli W277 and W306)
required for photolyase activity (Hsu et al 1996). Hsu et al therefore proposed that
these cry proteins might function as blue-light photoreceptors in humans. One
P1: FHN/fgi
P2: FHN
September 20, 1999
44
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
indirect piece of evidence supporting this hypothesis is the reported interaction
of the C-terminal portion of human cry2 (hcry2) with the serine/threonine phosphatase PP5, a protein similar to the RdgC protein phosphatase involved in signal
transduction arising from rhodopsin photoexcitation (Zhao & Sancar 1997).
More direct evidence came from the work of Miyamoto & Sancar (1998), who
showed that the mouse homologues (mCRY1 and mCRY2) of the human genes
are expressed in the mouse retina. Furthermore, mCRY1 expression oscillates in a
circadian rhythm within the superchiasmatic nucleus (SCN) where the master clock
is thought to reside. This work, together with the observation that the Arabidopsis
cry proteins participate in light signaling for circadian rhythms and photoperiodic
timing (see above), led to the proposal that these newly identified mammalian
photoreceptors set the phase of the circadian clock (Miyamoto & Sancar 1998).
To test the function of mcry1 more directly, a mouse mutant lacking this gene
was developed and found to have a phenotype consistant with a mcry1 role in
modulating circadian photoreception. This phenotype included reduced photic
sensitivity, enhanced phase shifts in response to light pulses, and, as with an
Arabidopsis cry1 mutant (Millar et al 1995), lengthening of the free-running period
(Thresher et al 1998).
More recently a single CRY gene has also been cloned from D. melanogaster.
Like mCRY1, its protein product appears to have a role in resetting the circadian
rhythm (Emery et al 1998). A mutant in this gene, cryb, fails to show a phase shift
in response to short intense light pulses. The cryb mutation shows a synergistic
effect on disruption of circadian rhythms in a norA (blind) background, suggesting
that the clock receives input from both mcry1 and rhodopsin. This mutation also
disrupts the cycling of the molecular clock components PER and TIM in certain
tissues. However, in contrast to the Arabidopsis and mouse cry mutations, there
was no lengthening of the free-running period (Stanewsky et al 1998).
?
PHOTOTROPIN (nph1) AND RELATED PROTEINS
Phototropin (nph1)
Just over a decade ago, Gallagher et al (1988) first reported the blue-light-activated
phosphorylation of a plasma membrane protein in etiolated pea seedlings. Short
and Briggs (1994) discussed the known biochemical and photobiological properties
of this system, so they are only briefly summarized here. The protein is likely to
be ubiquitous in higher plants, ranging from 114 to 130 kDa, depending upon
the species. The phosphorylation reaction can be driven both in vivo and in vitro,
the latter providing a convenient assay for biochemical characterization. Light
activates the kinase function rather than exposing sites for phosphorylation through
a protein conformational change. Phosphorylation occurs on multiple serine and
threonine residues. Although the reaction requires Mg+2, it is Ca+2 independent. It
is highly ATP specific, and the protein itself has an ATP-binding site. It can function
as a dimer, and the phosphorylation is at least partially intermolecular between the
dimer partners. The system also possesses a biochemical memory for a light pulse
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
45
at least in vitro where light activation of phosphorylation can be detected after
several minutes of darkness following the light treatment.
A great deal of physiological evidence suggests that the light-induced phosphorylation of the plasma membrane protein is involved in phototropism (see Short
& Briggs 1994): it occurs in the most photosensitive tissue, its action spectrum
matches that for phototropism, it is sufficiently fast to precede curvature development, it shows the same dark-recovery kinetics as phototropism following a saturating light pulse, and both the phosphorylation and phototropism obey the rules for
first order photochemistry. Finally, Salomon et al (1997a,b) have demonstrated a
gradient in in vivo phosphorylation across coleoptiles unilaterally illuminated by
certain fluences of blue light, with more phosphorylation on the illuminated than
the shaded side. These results are the first demonstration of a light-induced biochemical gradient that might be directly associated with phototropism. Studies
with Arabidopsis mutants (Liscum & Briggs 1995) provided direct genetic evidence that the phosphoprotein plays a central role in phototropism. Certain mutants
null for a phototropic response in growing organs from both light-grown and darkgrown seedlings (including the negative phototropic response of roots) showed no
light-induced phosphorylation and completely lacked the plasma membrane phosphoprotein. The mutant locus was designated nph1 (non-phototropic hypocotyl),
and the authors hypothesized that the nph1 protein might be the photoreceptor for
phototropism.
Photobiological and mutant studies showing differences in Arabidopsis phototropic responses to blue and green light (Konjević et al 1989, 1992) led the Poff
group to propose that there were two separate photoreceptors coupled in some
fashion. Because nph1 null mutants lack phototropic sensitivity to both blue and
green light, Liscum & Briggs (1995) presented instead a model involving a single
photoreceptor with two chromophores, perhaps analogous to the situation with the
DNA photolyases.
Although there was indirect evidence to suggest that the nph1 protein itself is
a photoreceptor, substrate, and kinase for the phosphorylation reaction (Short &
Briggs 1994), it remained a possibility that the three functions could be ascribed
to two or even three different polypeptides. The cloning and sequencing of Arabidopsis NPH1 (Huala et al 1997) resolved the kinase question: the nph1 protein
(996 amino acids) contained all 11 of the signature sequences for serine-threonine
kinases (Hanks & Quinn 1991, Hanks et al 1988). Mutations in the sequences
of three nph1 alleles confirmed that the NPH1 gene encoded the phosphoprotein.
The nph1 protein itself is hydrophilic and lacks any membrane-spanning domains
(Huala et al 1997). The association with the plasma membrane is most likely
through some sort of hydrophobic interaction, but the biochemical nature of the
association is currently unknown.
Three slightly smaller homologues of Arabidopsis nph1, two closely related
ones from Avena sativa (Zacherl et al 1998) and one from Zea mays (GenBank
Accession No. AF033263), were subsequently cloned. These proteins showed
more than 70% identity with the Arabidopsis protein, and more than 85% identity
over the two related domains designated LOV1 and LOV2 (for light, oxygen, and
?
P1: FHN/fgi
P2: FHN
September 20, 1999
46
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
voltage (see below). As predicted by earlier biochemical studies (Reymond et al
1992), the maize and oat proteins were significantly smaller than the Arabidopsis
protein, lacking several stretches of amino acids in the N-terminal domain. Partial
nph1 sequences have also been reported for pea (Lin et al 1991, Lin & Watson
1992), ice plant (Mesembryanthemum crystallinum) (Bauer et al 1994), and spinach
(GenBank Accession No. X73298).
Christie et al (1998) resolved the photoreceptor question by demonstrating that
nph1 expressed in insect cells grown in the dark exhibited blue-light-activated
phosphorylation with the same fluence dependence and kinetics as the native Arabidopsis protein. Large amounts of FMN accumulated in the insect cells producing
nph1, which led the authors to propose that FMN was the chromophore for the reaction. As the fluorescence excitation spectrum of the recombinant protein closely
matched the action spectrum for phototropism (Baskin & Iino 1987), Christie et al
concluded that nph1 was the photoreceptor for this response. An argument frequently expressed in favor of a carotenoid rather than a flavin as the chromophore
of a blue-light photoreceptor was that flavoprotein absorption spectra lack the sharp
peaks in the blue found in typical cryptochrome-type action spectra. However, such
fine structure is typically found in the absorption spectra of carotenoids (Quiñones
et al 1996). Evidently the binding of FMN to the NPH1 apoprotein provides a restricted hydrophobic environment that leads to its more sharply defined absorption
spectrum and hence the characteristic action spectrum for phototropism. Rüdiger
& Briggs (1995) found that the thio reagent N-phenylmaleimide was far more
effective in inhibiting light-induced phosphorylation than the more hydrophilic Nethylmaleimide, consistent with this model. Christie et al (1999) have designated
the holoprotein nph1 phototropin.
A recent paper from the Cashmore laboratory (Ahmad et al 1998b) reported that
cry1cry2 double mutants failed to show first positive phototropic curvature and that
membrane preparations failed to show blue-light-induced phosphorylation. This
evidence suggested that the cryptochromes were involved in phototropism. However, Lascève et al (1999) have shown that such double mutants (as well as cry1 and
cry2 single mutants) respond to blue-light fluences inducing first positive curvature
over the same fluence range as wild-type. In addition, the double mutants exhibited wild-type sensitivity for blue-light-induced phosphorylation. Both single and
double mutants showed somewhat reduced curvature, but no difference from wild
type in threshhold, peak, or saturation fluences. Hence in the absence of either
CRY protein, the seedlings still detected and responded to light direction, and the
role of the CRY proteins, if any, must be downstream of nph1. It is possible that the
protocol used by Ahmad et al (1998b) did not optimize the very weak first positive phototropic response of Arabidopsis, accounting for the differences observed.
?
The LOV Domains
A sequence found both in the N-terminal and central regions of the nph1 protein
is also present in proteins from an extremely diverse group of organisms. This domain, found in light sensors, oxygen sensors, and the eag family of voltage-gated
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
47
potassium channel proteins, has been designated LOV, as mentioned above (Huala
et al 1997). Light sensors carrying a LOV domain include nph1 (Huala et al
1997) and white collar 1 (WC-1), a putative blue-light sensor from the filamentous fungus Neurospora crassa that is required for blue-light-dependent responses
(Ballario et al 1996). Oxygen sensors include a bacterial regulator of nitrogen fixation (NIFL) (reviewed by Dixon 1998), an aerotaxis protein (AER) from E. coli
(Bibikov et al 1997), a methyl-accepting protein from Desulfovibrio vulgaris
(Fu et al 1994), and a regulator of bacteriorhodopsin production (BAT) from
Halobacterium halobium (Gropp & Betlach 1994, Shand & Betlach 1991). The
eag potassium channel family group includes a large number of eag, elk, and erg
subunit proteins from Drosophila and a variety of vertebrates. Although the activity of these potassium channels is voltage-regulated, one member of the family, the
HERG (human ether-a-gogo related gene) potassium channel was recently found
to be oxygen-regulated (Tagliatela et al 1997).
The LOV domain sequence bears some similarity to a PAS domain (Zhulin
& Taylor 1997), but there is a much higher degree of sequence similarity among
LOV domains than between LOV and PAS domains (Huala et al 1997, Ballario
et al 1998), suggesting that LOV domains form a subgroup within the larger group
of PAS domains. Like PAS domains, LOV domains can interact to form dimers
(Ballario et al 1998). In the WC-1 protein, the LOV domain alone is capable of
forming a dimer with another LOV domain but can dimerize only very weakly with
a PAS domain. In addition, three mutations isolated in the WC-1 LOV domain
failed to prevent LOV dimerization but still blocked WC-1 function, implying an
additional function for this domain (Ballario et al 1998). Unlike the WC-1 protein,
the isolated LOV domain from the human potassium channel gene HERG does
not multimerize (Cabral et al 1998). Interestingly, a human mutation (11261) in
which only the portion of HERG containing the LOV domain is expressed results
in a dominant phenotype of sudden cardiac death in juveniles as well as adults.
This appears to result from the assembly of mutant and wild-type subunits into an
abnormal potassium channel which results in a heart malfunction in heterozygous
individuals (Li et al 1997).
LOV domains can also serve as flavin-binding sites. Of the proteins containing
a LOV domain, NIFL from Azotobacter vinelandii was the first to be identified as
a flavoprotein containing FAD (Hill et al 1996). More recently, Söderbäck et al
(1998) narrowed the FAD-binding domain to the N-terminal 284 amino acids, a
region spanning the NIFL LOV domain. Since then, AER has also been shown
to bind FAD (Bibikov et al 1997, Grishanin & Bibikov 1997, Rebbapragada et al
1997). The sequence similarity between NIFL, AER and nph1 in the region of
the LOV domains led to the hypothesis that they might function as flavin-binding
sites (Huala et al 1997, Rebbapragada et al 1997).
Christie et al (1999) have recently demonstrated that the LOV domains of nph1
are capable of binding the flavin FMN when expressed as isolated domains in a
heterologous expression system. When they expressed either LOV1 or LOV2 separately in E. coli and purified the recombinant peptide, they found that both LOV1
and LOV2 domains bound FMN with a flavin/protein ratio of 1. Expression of a
?
P1: FHN/fgi
P2: FHN
September 20, 1999
48
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
larger construct containing both LOV domains bound FMN with a ratio approaching 2. These findings are consistent with the two-chromophore model proposed
by Liscum & Briggs (1995).
The crystal structure of the human HERG LOV domain, known as the eag
domain, has been determined (Cabral et al 1998), as well as the FixL LOV domain for Bradyrhizobium japonicum (Gong et al 1998). In both cases, the LOV
domain forms a ß-sheet core flanked by α-helices making a hydrophobic pocket
that is capable of binding a ligand. The hydrophobic pocket is accessible for
ligand binding via a hydrophilic entryway. In the case of the FixL domain, the
ligand is known to be a heme, although the authors point out that the structure is
sufficiently flexible to accommodate a range of cofactors. The PAS domains from
the bacterial photoactive yellow protein (PYP) form similar structures (Genick
et al 1997, Pellequer et al 1998). As with the HERG and FixL LOV domains,
a ß-sheet core flanked by α-helices is also involved, but in this case the ligand
is a 4-hydroxycinnamyl chromophore, present in the hydrophobic pocket of the
barrel-like structure in both ground and photoactivated states (Genick et al 1997).
This pocket-containing structure is also found in other flavin-binding domains
(Liepinsh et al 1998), although in these cases there is little homology with the
LOV domains (WR Briggs & E Huala, unpublished observations).
?
Components of Signal Transduction Downstream
from Phototropin
Unlike the case with the cryptochromes, mutations at the NPH1 locus do not show
a pleiotropic phenotype. They are impaired in all of their phototropic responses,
but there are no other obvious differences from wild-type. In their mutant studies,
Liscum & Briggs (1995, 1996) report three additional loci affecting phototropism.
For two of these loci, designated NPH2 and NPH3, normal levels of phototropin
are present, and nph1 phosphorylation proceeds normally, although phototropism
is impaired. Hence the gene products encoded by these loci must act downstream
from nph1 in the phototropism signal transduction pathway. The third additional
locus, NPH4, is impaired in both phototropism and gravitropism (Liscum & Briggs
1996). NPH4 appears to be a conditional modulator of auxin-induced differential
growth responses and is deficient in auxin-induced gene expression (Stowe-Evans
et al 1998). The identification of the NPH2, NPH3, and NPH4 genes and the
functions of their encoded proteins should enhance our knowledge of signal transduction in phototropism.
Arabidopsis NPL1 and Adiantum PHY3
Jarillo et al (1998) recently reported a NPH1 homologue in Arabidopsis designated
NPL1 (NPHl-like) that encodes a protein slightly shorter than nph1 (916 amino
acids compared with 996 for nph1). It shows approximately 58% identity and
67% similarity with nph1 at the amino acid level and contains LOV1, LOV2,
and kinase domains. Although it is expressed (having been cloned from a cDNA
library), its role as a photoreceptor is not clear. Null mutants at the NPH1 locus fail
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
49
to show any phototropic curvature (Liscum & Briggs 1995, Lascève et al 1999),
though presumably NLS1 is being expressed. Alternatively, perhaps phototropism
depends upon a functional dimer (Reymond et al 1992) that requires both gene
products, possibly because in the absence of one protein the other is unstable or
inactive. This explanation seems unlikely as the nph1 protein expressed in insect
cells shows normal stability and photoactivity in the absence of any other plant
proteins (Christie et al 1998).
Nozue et al (1998) recently described a remarkable gene from the fern Adiantum
capillus-veneris (AcPHY3) that encodes a protein with properties of two different
photoreceptors: phytochrome and nph1. The amino-terminal 564 amino acids
show 52% identity with Arabidopsis phyA and include the signature phytochrome
chromophore-binding domain. When the authors expressed the full-length apoprotein in yeast and added the chromophore phycocyanobilin, the chromophore became bound as assayed by zinc-blot analysis, and showed typical phytochrome
photoreversibility as determined by a Pr minus Pfr difference spectrum. However,
the similarity with phytochrome in the N-terminal region abruptly ends at amino
acid 564. Dimerization domains and other features of a typical phytochrome are
missing, and instead, the C-terminal portion of the protein shows 57% identity with
Arabidopsis nph1, including both LOV domains and the complete serine/threonine
kinase domain. As with the LOV domains from Arabidopsis and oat nph1, those
from Adiantum protein also bind FMN tightly (Christie et al 1999). Given the
complexity of fern responses to both blue and red light (reviewed by Wada &
Sugai 1994), it is tempting to hypothesize that PHY3 plays a photoreception role
in both red and blue spectral regions. It will be interesting to see whether the
PHY3 protein undergoes light-activated phosphorylation. If so, are both blue and
red light able to induce it? Alternatively, does red light transformation of the
phytochrome domain to its Pfr form alter the properties of the phosphorylation
reaction? Further, does phosphorylation affect phytochrome phototransformation
properties in any way?
?
A BLUE-LIGHT PHOTORECEPTOR FOR STOMATA
It has been known for two decades that very small amounts of blue light induce
the opening of stomata via excitation of a photoreceptor that is independent of
photosynthesis or phytochrome (Iino et al 1985, Travis & Mansfield 1981; see
Zeiger & Zhu 1998b for review). The swelling of isolated guard cell protoplasts in
response to blue light indicated that the blue-light photoreceptors were located in
the guard cells (Zeiger & Hepler 1977). However, the nature of the photoreceptor
remained unknown. An action spectrum by Karlsson (1986) showed fine structure
in the blue region of the cryptochrome type, but because measurements were made
only as far as 370 nm, little could be said about the presence or absence of a broad
action band in the UV-A region.
Guard cells contain a functional xanthophyll cycle (Masamoto et al 1993),
considered to be an important component for photoprotection in chloroplasts
P1: FHN/fgi
P2: FHN
September 20, 1999
50
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
(Demmig-Adams & Adams 1992). However, there is a close correlation between
fluence rate and the concentration of guard cell zeaxanthin, a relationship not
found in mesophyll cells (Srivastiva & Zeiger 1995a). Dithiothreitol (DTT)
inhibits zeaxanthin formation (Yamamoto & Kamite 1972) and inhibits stomatal opening induced by blue light but not by red (Srivastiva & Zeiger 1995b).
This and other correlative evidence implicating zeaxanthin as the photoreceptor
for blue-light-activated stomatal opening has been reviewed by Zeiger & Zhu
(1998b).
Recently, Niyogi et al (1998) described an Arabidopsis mutant, npq1 (for nonphotochemical quenching) that lacks violaxanthin de-epoxidase and is therefore
unable to convert violaxanthin to zeaxanthin (a reaction normally occurring under high light conditions). Zeiger & Zhu (1998a, 1998b) tested the responses
of wild-type and npq1 mutant seedlings for blue-light-induced stomatal opening
under increasing background levels of red light. Wild-type stomata showed a
strong increase in the blue-light-activated opening response with an increasing
fluence rate of the background red light. By contrast, the npq1 mutant showed no
changes in stomatal aperture regardless of the red-light fluence rate. These experiments support the hypothesis that zeaxanthin may play a role as the photoreceptor
chromophore for stomatal opening.
?
CO-ACTION BETWEEN PHOTORECEPTOR SYSTEMS
A response to photoexcitation of one plant pigment system may be modified by
photoexcitation of another, or indeed may even require this additional photoexcitation for full expression of a given response (for a detailed review, see Mohr 1994).
Here we consider recent papers in which studies with mutants have elucidated
these relationships with respect to specific photoreceptors.
The Phototropism Pathway
Phytochrome phototransformation in dark-grown seedlings dramatically alters
phototropic responses to blue light, sometimes in an extremely complex manner (Iino 1990). First positive curvatures of coleoptiles are de-sensitized, whereas
second positive curvatures are sensitized (see Briggs 1963a). By contrast, there
is no change in sensitivity of Arabidopsis hypocotyls (threshold fluences are unchanged), but the magnitude of the curvature response is enhanced (Parks et al
1996). Partial photoreversibility by far-red light (coleoptiles: Briggs 1963b;
Arabidopsis: Janoudi & Poff 1992) and an action spectrum for the effect (coleoptiles: Chon & Briggs 1966; Arabidopsis: Janoudi & Poff 1992) established that
this response is phytochrome mediated.
Studies with phytochrome mutants showed that phyA is responsible for the
red light enhancement of first positive phototropism at low fluences of red light
(Janoudi et al 1997, Parks et al 1996), that phyB played no role, and that the
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
51
response to higher fluences of red light must be through some phytochrome other
than phyA or phyB (Parks et al 1996). For the enhancement observed in Arabidopsis for second positive curvature, however, both phyA and phyB played
an important role (Hangarter 1997). Both cry1cry2 and phyAphyB double mutants show reduced first positive curvature, making it possible that the CRY and
PHY photoreceptors play a role in determining the magnitude of the phototropic
response. Alternatively, physiological differences arising from differences in conditions for seed development and harvest between mutants and wild-type could
account for the reduction (Lascève et al 1999).
?
Cryptochrome Pathways
Interactions between cry1- and phyA- or phyB-activated signal transduction pathways are complex. A number of studies have investigated potential interactions as
revealed by single, double, and triple mutants at the CRY1, PHYA, and PHYB loci.
Results differ widely depending upon growth conditions, frequency and/or duration of red- or blue-light treatments, the fluence rates used, and the response studied
(seed germination, inhibition of hypocotyl elongation, hook opening, cotyledon
unfolding, cotyledon expansion, or anthocyanin synthesis). Under some conditions
cry1 can function independently of the phytochromes, but under other conditions
its action depends heavily on one or the other or both. Space limitations preclude
a detailed discussion of these studies, and the reader is referred to recent articles
and references therein for further information and analysis (Ahmad & Cashmore
1997, Casal & Boccalandro 1995, Casal & Mazzella 1998, Neff & Chory 1998,
Poppe et al 1998). As yet there are no studies that have included cry2 mutants.
Furthermore, other phytochromes function even in the absence of phyA and phyB
(Poppe & Schäfer 1997), and their role in blue-light-activated signal transduction
remains unexplored.
Yeh & Lagarias (1998) recently demonstrated light-regulated autophosphorylation of eukaryotic phytochrome, indicating that phytochrome could function as
a protein kinase. Ahmad et al (1998c) used purified recombinant photoreceptors
to investigate whether cry1 serves in vitro as a substrate for phyA-mediated phosphorylation. Cry1 alone showed no phosphorylation in response to light. In the
presence of phyA in the dark, there was limited phosphorylation of both cry1 and
phyA, but both red and blue light increased the phosphorylation of both photoreceptors (phosphorylation was also reported following far-red light, but the results
were not shown, nor was far-red reversibility tested). Cry1 phosphorylation was
localized to serine residues in the C-terminal domain. Similar results were obtained with recombinant cry2. In addition, yeast two-hybrid studies indicated a
direct interaction between the C-terminal domains of cry1 and phyA. Moreover,
in vivo phosphorylation studies showed Pfr-dependent phosphorylation of cry1.
However, it is premature to speculate as to how these intriguing findings relate to
the physiological studies mentioned above, although they provide the first evidence
for a possible direct interaction between photoreceptors.
P1: FHN/fgi
P2: FHN
September 20, 1999
52
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
ACTION SPECTRA AND NOMENCLATURE
A curious paradox arises from the use of the term cryptochrome for the photolyaselike blue-light photoreceptors. Gressel specifically used it to designate a photoreceptor for which the action spectrum resembles the absorption spectrum of a
flavoprotein or a cis-carotene (known to have a small absorption in the UV-A).
At least for cry1, it seems unlikely that this action-spectrum requirement is met.
Both Ahmad & Cashmore (1993) and Young et al (1992) show strong UV-A action
in the suppression of hypocotyl elongation in Arabidopsis mutants lacking cry1
despite virtually complete loss of sensitivity to blue light. Hence, only a fraction of
the UV-A sensitivity may be related to cry1. Indeed, the UV-sensitivity found both
in the mutant and wild-type seedlings is likely to be mediated through a specific
UV-A sensor and not through cry1 (Young et al 1992). Cry1 must contribute some
UV-A photosensitivity, however, as cry1 overexpression either in tobacco (Lin et al
1995a) or Arabidopsis (Lin et al 1996a) results in increased sensitivity to UV-A.
With pterin-type photolyases, the pterin largely determines the action spectrum
for light-driven DNA repair (Sancar et al 1987). Absorbed light energy is transferred to the FAD chromophore that initiates the repair reaction. The FAD itself
can act as a chromophore for the reaction, but is approximately tenfold less effective (Jorns et al 1987, 1990). The pterin in vivo normally absorbs largely in the
UV-A, but Malhotra et al (1994) have described a photolyase from Bacillus firmus
that shows action well into the blue region of the spectrum. If the cryptochromes
utilize a photochemical mechanism that is similar to that used by the photolyases,
then the action spectra for cryptochrome-dependent processes would be expected
to reflect largely the pterin absorption spectrum and not that of the FAD. The action
of green light could still be ascribed to the semiquinone form of FAD, as proposed
by Lin et al (1995b). The limited information available for the action spectrum for
suppression of hypocotyl elongation in Arabidopsis suggests that this may be the
case. A careful action spectrum for wild-type, cry1, cry2, and cry1cry2 mutant
seedlings of Arabidopsis is clearly needed to clarify the situation.
The following paradox has arisen: If the above hypothesis is correct, then
cryptochromes that do not yield a cryptochrome-type action spectrum have preempted the cryptochrome name. Phototropin, with its cryptochrome-type action
spectrum, but absolutely no structural similarity to the cryptochromes and with a
different chromophore can not be called a cryptochrome!
?
THE NEXT STEPS
Although astonishing progress has been made over the past several years in characterizing blue-light photoreceptors such as cry1, cry2, the animal cryptochromes,
and phototropin, much remains to be done. A present there is little or no information about events in the signal transduction cascades immediately downstream
from the various photoreceptors. At this writing, not a single interacting reaction
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
53
partner has been identified. Once such partners have been identified, it will be necessary to determine in what way(s) chromophore excitation leads to downstream
events. With the plant photoreceptors, there is increasing evidence of participation of the COP9 nuclear complex in signal transduction both for cryptochromeand phytochrome-elicited processes. However, how the specificity for the various
responses is retained downstream of these complexes remains a mystery. The current list of plant photoreceptors responding to blue and ultraviolet light is not likely
to be complete. Beyond the identification of a putative chromophore in the stomatal guard cells, there is still much to learn. What is the nature of the interactions
detected between pathways that are activated by different photoreceptors? Why are
there interactions between two photoreceptors in some cases, but independent action in other cases? What is the nature of this signaling network? As mentioned
above, there is evidence for a specific UV-A photoreceptor independent of cry1,
and there is also strong evidence for at least one UV-B photoreceptor (Teramura
1996, Christie & Jenkins 1996). What are these photoreceptors? How are the
various photoreceptors coupled to the circadian oscillator? The coming decade
will offer some exciting advances as we probe for a deeper understanding of photomorphogenesis in plants and the photobiological aspects of circadian rhythms
in both plants and animals.
?
ACKNOWLEDGMENTS
The authors are extremely grateful to Drs. John M Christie, Margaret A Olney,
and Richard Walden for their careful review of this manuscript. Work discussed
from the senior author’s laboratory was generously supported by the National
Science Foundation. This is Carnegie Institution of Washington Department of
Plant Biology Publication No. 1413.
Visit the Annual Reviews home page at www.AnnualReviews.org
LITERATURE CITED
Adams MD, Kerlavage AR, Fleischmann RD,
Fuldner RA, Bult CJ, et al. 1995. Initial assessment of human gene diversity
and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature
377(6547 Suppl):3–174
Ahmad M, Cashmore AR. 1993. HY4 gene of A.
thaliana encodes a protein with the characteristics of a blue-light photoreceptor. Nature
366:162–66
Ahmad M, Cashmore AR. 1996. Seeing blue:
the discovery of cryptochrome. Plant Mol.
Biol. 30:851–61
Ahmad M, Cashmore AR. 1997. The bluelight receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J.
11:421–27
Ahmad M, Jarillo JA, Cashmore AR. 1998a.
Chimeric proteins between cry1 and cry2
Arabidopsis blue-light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell 10:197–207
Ahmad M, Jarillo JA, Klimczak LJ, Landry LG,
Peng T, et al. 1997. An enzyme similar to animal type II photolyases mediates photore-
P1: FHN/fgi
P2: FHN
September 20, 1999
54
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
activation in Arabidopsis. Plant Cell 9:199–
207
Ahmad M, Jarillo JA, Smirnova O, Cashmore
AR. 1998b. Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature 392:720–23
Ahmad M, Jarillo JA, Smirnova O, Cashmore
AR. 1998c. The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1:939–48
Ahmad M, Lin C, Cashmore AR. 1995.
Mutations throughout an Arabidopsis
blue-light photoreceptor impair blue-lightresponsive anthocyanin accumulation and
inhibition of hypocotyl elongation. Plant J.
8:653–58
Ang L-H, Deng X-W. 1994. Regulatory hierarchy of photomorphogenic loci: allelespecific and light-dependent interaction between the HY5 and COP1 loci. Plant Cell
6:613–28
Bagnall DJ, King RW, Hangarter RP. 1996.
Blue-light promotion of flowering is absent in hy4 mutants of Arabidopsis. Planta
200:278–80
Ballario P, Talora C, Galli D, Linden H, Macino
G. 1998. Roles in dimerization and blue light
photoresponse of the PAS and LOV domains
of Neurospora crassa white collar proteins.
Mol. Microbiol. 29:719–29
Ballario P, Vittorioso P, Magrelli A, Talora C,
Cabibbo A, et al. 1996. White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J. 15:
1650–57
Baskin TI, Iino M. 1987. An action spectrum in
the blue and ultraviolet for phototropism in
alfalfa. Photochem. Photobiol. 46:127–36
Batschauer A. 1993. A plant gene for photolyase: an enzyme catalyzing the repair of
UV-light-induced DNA damage. Plant J. 4:
705–9
Batschauer A. 1998. Photoreceptors of higher
plants. Planta 206:479–92
Batschauer A, Rocholl M, Kaiser T, Nagatani
A, Furuya M, et al. 1996. Blue and UV-A
light-regulated CHS expression in Arabidop-
sis independent of phytochrome A and phytochrome B. Plant J. 9:63–69
Bauer B, Fischer K, Winter K, Dietz K-J. 1994.
cDNA sequence of a protein kinase from
the inducible crassulacean acid metabolism
plant Mesembryanthemum crystallinum L.,
encoding a SNF-1 homolog. Plant Physiol.
106:1225–26
Bibikov SI, Biran R, Rudd KE, Parkinson JS.
1997. A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol. 179:4075–79
Blum DE, Neff MM, Van Volkenburgh E.
1994. Light-stimulated cotyledon expansion
in the blu3 and hy4 mutants of Arabidopsis
thaliana. Plant Physiol. 105:1433–36
Briggs WR. 1963a. The phototropic responses
of higher plants. Annu Rev. Plant Physiol.
14:311–352
Briggs WR. 1963b. Red light, auxin relationships, and the phototropic responses of corn
and oat coleoptiles. Am. J. Bot. 50:196–207
Briggs WR, Iino M. 1983. Blue-light-absorbing
photoreceptors in plants. Phil. Trans. R. Soc.
London Ser. B 303:347–59
Briggs WR, Tocher RD, Wilson JF. 1957. Phototropic auxin redistribution in corn coleoptiles. Science 126:210–12
Butler WL, Norris KH, Siegelman HA, Hendricks SB. 1959. Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants.
Proc. Natl. Acad. Sci. USA 45:1703–8
Cabral JHM, Lee A, Cohen SL, Chait BT, Li
M, et al. 1998. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eucaryotic PAS domain.
Cell 95:649–55
Casal JJ, Boccalandro H. 1995. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta 197:213–18
Casal JJ, Mazzella MA. 1998. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA,
PhyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 118:19–
25
Cashmore AR. 1997. The cryptochrome family
?
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
of photoreceptors. Plant Cell Environ. 20:
764–67
Cashmore AR, Jarillo JA, Wu Y-J, Liu D.
1999. Cryptochromes: blue light receptors
for plants and animals. Science 284:760–65
Chamovitz DA, Deng X-W. 1996. Light signaling in plants. Crit. Rev. Plant Sci. 15:455–78
Chamovitz DA, Wei N, Osterlund MT, von
Arnim AG, Staub JM, et al. 1996. The COP9
complex, a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 86:115–21
Cho MH, Spalding EP. 1996. An anion channel
in Arabidopsis hypocotyls activated by blue
light. Proc. Natl. Acad. Sci. USA 93:8134–38
Chon HP, Briggs WR. 1966. Effect of red light
on the phototropic sensitivity of corn coleoptiles. Plant Physiol. 41:1217–24
Christie JM, Jenkins GI. 1996. Distinct UVB and UV-A/Blue light signal transduction
pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell
8:1555–67
Christie JM, Reymond P, Powell GP,
Bernasconi P, Raibekas AA, et al. 1998.
Arabidopsis NPH1: A flavoprotein with the
properties of the photoreceptor for phototropism. Science 282:1698–701
Christie JM, Salomon M, Nozue K, Wada M,
Briggs WR. 1999. LOV (light, oxygen, or
voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites
for the chromophore flavin mononucleotide.
Proc. Natl. Acad. Sci. USA. 96:8779–83
Conley TR, Shih M-C. 1995. Effects of light
and chloroplast functional state on expression of nuclear genes encoding chloroplast
glyceraldehyde-3-phosphate dehydrogenase
in long hypocotyl (hy) mutants and wildtype Arabidopsis thaliana. Plant Physiol.
108:1013–22
Cosgrove DJ. 1981. Rapid suppression of
growth by blue light. Plant Physiol. 67:584–
90
Darwin C. 1881. The Power of Movement in
Plants (Da Capo Press Reprint Ed., 1966).
New York: Da Capo
55
Demmig-Adams B, Adams WW III. 1992. Photoprotection and other responses of plants to
high light stress. Annu. Rev. Plant Physiol.
Plant Mol. Biol. 43:599–626
Dixon R. 1998. The oxygen-responsive NIFLNIFA complex: a novel two-component
regulatory system controlling nitrogenase
synthesis in gamma-proteobacteria. Arch.
Microbiol. 169:371–80
Emery P, So WV, Kaneko M, Hall JC, Rosbash
M. 1998. CRY, a Drosophila clock and lightregulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95:669–79
Fankhauser C, Chory J. 1997. Light control of
plant development. Annu. Rev. Cell Dev. Biol.
13:203–29
Flint LH. 1934. Light in relation to germination and dormancy in lettuce seeds. Science
80:38–40
Flint LH, McAlister ED. 1935. Wavelengths
of radiation in the visible spectrum inhibiting the germination of light-sensitive lettuce
seeds. Smithson. Misc. Collect. 94(5):1–11
Flint LH, McAlister ED. 1937. Wavelengths
of radiation in the visible spectrum promoting the germination of light-sensitive lettuce
seeds. Smithson. Misc. Collect. 96(2):1–11
Fu RO, Wall JD, Voordouw G. 1994. DcrA,
a c-type heme-containing methyl-accepting
protein from Desulfovibrio vulgaris Hildenborough, senses the oxygen concentration or
redox potential of the environment. J. Bacteriol. 176:344–50
Fuglevand G, Jackson JA, Jenkins GI. 1996.
UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in
Arabidopsis. Plant Cell 8:2347–57
Gaba V, Black M. 1979. Two separate photoreceptors control hypocotyl growth in green
seedlings. Nature 278:51–53
Gallagher S, Short TW, Pratt LH, Ray PM,
Briggs WR. 1988. Light-induced changes in
two proteins found associated with plasma
membrane fractions from pea stem sections.
Proc. Natl. Acad. Sci. USA 85:8003–7
?
P1: FHN/fgi
P2: FHN
September 20, 1999
56
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
Galland P, Senger H. 1988. The role of pterins in
the photoreception and metabolism of plants.
Photochem. Photobiol. 48:811–20
Galston AW. 1949. Riboflavin-sensitized photooxidation of indoleacetic acid and related
compounds. Proc. Natl. Acad. Sci. USA 35:
10–17
Galston AW. 1950. Riboflavin, light, and the
growth of plants. Science 111:619–24
Genick UK, Borgstahl GEO, Ng K, Ren Z,
Pradervand C, et al. 1997. Structure of a
protein photocycle intermediate by millisecond time-resolved crystallography. Science
275:1471–75
Gong W, Hao B, Mansy SS, Gonzalez G, GillesGonzalez MA, et al. 1998. Structure of a biological oxygen sensor: a new mechanism for
heme-driven signal transduction. Proc. Natl.
Acad. Sci. USA 95:15177–82
Gressel J. 1977. Blue light photoreception. Photochem. Photobiol. 30:749–54
Grishanin RN, Bibikov SI. 1997. Mechanisms
of oxygen taxis in bacteria. Biosci. Rep. 17:
77–83
Gropp F, Betlach MC. 1994. The bat gene of
Halobacterium halobium encodes a transacting oxygen inducibility factor. Proc. Natl.
Acad. Sci. USA 91:5475–79
Guo H, Yang H, Mockler TC, Lin C. 1998. Regulation of flowering time by Arabidopsis photoreceptors. Science 279:1360–63
Hangarter RP. 1997. Gravity, light and
plant form. Plant Cell Environ. 20:796–
800
Hanks SK, Quinn AM. 1991. Protein kinase
catalytic domain sequence database: identification of conserved features of primary
structure and classification of family members. Methods Enzymol. 200:38–62
Hanks SK, Quinn AM, Hunter T. 1988. The protein kinase family: conserved features and
deduced phylogeny of the catalytic domains.
Science 241:42–52
Hill S, Austin S, Eydmann T, Jones T, Dixon R.
1996. Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-
sensitive switch. Proc. Natl. Acad. Sci USA
93:2143–48
Hoffman PD, Batschauer A, Hays JB. 1996.
PHH1, a novel gene from Arabidopsis
thaliana that encodes a protein similar to
plant blue-light photoreceptors and microbial
photolyases. Mol. Gen. Genet. 253:259–65
Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang
R-P, et al. 1996. Putative human blue light
photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry 35:13871–77
Huala E, Oeller PW, Liscum E, Han I-S, Larsen
E, et al. 1997. Arabidopsis NPH1: A protein
kinase with a putative redox-sensing domain.
Science 278:2120–23
Iino M. 1990. Phototropism: mechanisms and
ecological implications. Plant Cell Environ.
13:633–50
Iino M, Ogawa T, Zeiger E. 1985. Kinetic properties of the blue-light response of stomata.
Proc Natl. Acad. Sci. USA 82:8019–23
Jackson JA, Jenkins GI. 1995. Extensiongrowth responses and expression of flavonoid
biosynthesis genes in the Arabidopsis hy4
mutant. Planta 197:233–39
Janoudi A-K, Gordon WR, Wagner D, Quail P,
Poff KL. 1997. Multiple phytochromes are
involved in red-light-induced enhancement
of first-positive phototropism in Arabidopsis
thaliana. Plant Physiol. 113:975–79
Janoudi A-K, Poff KL. 1992. Action spectrum for enhancement of phototropism by
Arabidopsis thaliana seedlings. Photochem.
Photobiol. 56:655–59
Jarillo JA, Ahmad M, Cashmore AR. 1998.
NPL1 (accession No. AF053941): a second
member of the NPH serine/threonine kinase
family of Arabidopsis (PGR 98–100). Plant
Physiol. 117:719
Jenkins GI. 1997. UV and blue light signal
transduction in Arabidopsis. Plant Cell Environ. 20:773–78
Jenkins GI, Christie JM, Fuglevand G, Long
JC, Jackson JA. 1995. Plant responses to UV
and blue light: biochemical and genetic approaches. Plant Sci. 112:117–38
Jorns MS, Baldwin ET, Sancar GB, Sancar
?
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
A. 1987. Action mechanism of Escherichia
coli DNA photolyase II. Role of the chromophores in catalysis. J. Biol. Chem. 262:
486–91
Jorns MS, Wang B, Jordan SP, Chanderkar LP.
1990. Chromophore function and interaction
in Escherichia coli DNA photolyase: reconstitution of the apoenzyme with pterin and/or
flavin derivatives. Biochemistry 29:552–61
Kanegae T, Wada M. 1998. Isolation and characterization of homologues of plant bluelight photoreceptor (cryptochrome) genes
from the fern Adiantum capillus-veneris.
Mol. Gen. Genet. 259:345–53
Karlsson PE. 1986. Blue light regulation of
stomata in wheat seedlings. II. Action spectrum and search for action dichroism. Physiologia Plantarum 66:207–10
Kaufman LS. 1993. Transduction of blue-light
signals. Plant Physiol. 102:333–37
Khurana JP, Kochhar A, Tyagi AK. 1998. Photosensory perception and signal transduction
in higher plants-molecular genetic analysis.
Crit. Rev. Plant Sci. 17:465–539
Konjević R, Khurana JP, Poff KL. 1992. Analysis of multiple photoreceptor pigments for
phototropism in a mutant of Arabidopsis
thaliana. Photochem. Photobiol. 55:789–
92
Konjević R, Steinitz B, Poff KL. 1989. Dependence of the phototropic response of Arabidopsis thaliana on fluence rate and wavelength. Proc. Natl. Acad. Sci. USA 86:9876–
80
Koornneef M, Rolff E, Spruit CJP. 1980. Genetic control of light-inhibited hypocotyl
elongation in Arabidopsis thaliana (L.)
Heynh. Z. Pflanzenphysiol. 100:147–60
Kwok SF, Piekos B, Miséra S, Deng X-W.
1996. A complement of ten essential
and pleiotropic Arabidopsis COP/DET/FUS
genes is necessary for repression of photomorphogenesis in darkness. Plant Physiol.
110:731–42
Lascève G, Leymarie J, Olney MA, Liscum E,
Christie JM, et al. 1999. Arabidopsis contains
at least four independent blue light-activated
57
signal transduction pathways. Plant Physiol.
120:605–14
Laskowski MJ, Briggs WR. 1989. Regulation
of pea epicotyl elongation by blue light.
Fluence-response relationships and growth
distribution. Plant Physiol. 89:293–98
Lewis BD, Karlin-Newman G, Davis RW,
Spalding EP. 1997. Ca2+-activated anion
channels and membrane depolarizations induced by blue light and cold in Arabidopsis
seedlings. Plant Physiol. 114:1327–34
Li X, Xu J, Li M. 1997. The human 11261 mutation of the HERG potassium channel results
in a truncated protein that contains a subunit
interaction domain and decreases the channel
expression. J. Biol. Chem. 272:705–8
Li YF, Sancar A. 1990. Active site of Escherichia coli DNA photolyase: Mutations
at Trp277 alter the selectivity of the enzyme
without affecting the quantum yield of photorepair. Biochemistry 29:5698–706
Liepinsh E, Kitamura M, Murakami T, Nakaya
T, Otting G. 1998. Common ancestry of serine proteases and flavin-binding domains.
Nat. Struct. Biol. 5:102–3
Lin C, Ahmad M, Cashmore AR. 1996a. Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant
J. 10:893–902
Lin C, Ahmad M, Chan J, Cashmore AR. 1996b.
CRY2: A second member of the Arabidopsis cryptochrome gene family (Accession
No. U43397) (PGR96–001). Plant Physiol.
110:1047
Lin C, Ahmad M, Gordon D, Cashmore AR.
1995a. Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results
in hypersensitivity to blue, UV-A, and green
light. Proc. Natl. Acad. Sci. USA 92:8423–27
Lin C, Cashmore AR. 1996. Cryptochrome and
plant photomorphogenesis. In Regulation of
Plant Growth and Development by Light, ed.
WR Briggs, RL Heath, EM Tobin, pp. 30–41.
Rockville, MD: Am. Soc. Plant Physiol.
Lin C, Robertson DE, Ahmad M, Raibekas AA,
Jorns MS, et al. 1995b. Association of flavin
?
P1: FHN/fgi
P2: FHN
September 20, 1999
58
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
adenine dinucleotide with the Arabidopsis
blue light receptor CRY1. Science 269:968–
70
Lin C, Yang H, Guo H, Mockler T, Chen J, et al.
1998. Enhancement of blue-light sensitivity
of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci.
USA 95:2686–90
Lin X, Feng X-H, Watson JC. 1991. Differential
accumulation of transcripts encoding protein
kinase homologs in greening pea seedlings.
Proc. Natl. Acad. Sci. USA 88:6951–55
Lin X, Watson JC. 1992. cDNA sequence of
PsPK5, a protein kinase homolog from Pisum
sativum L. Plant Physiol. 100:1072–74
Liscum E, Briggs WR. 1995. Mutations in
the NPH1 locus of Arabidopsis disrupt the
perception of phototropic stimuli. Plant Cell
7:473–85
Liscum E, Briggs WR. 1996. Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol. 112:291–96
Liscum E, Hangarter R. 1994. Mutational analysis of blue-light sensing in Arabidopsis.
Plant Cell Environ. 17:639–48
Lorenzi R, Ceccarelli N, Lercari B, Gualtieri
P. 1994. Identification of retinol in higher
plants: is a rhodopsin-like protein the blue
light photoreceptor? Phytochemistry 36:
599–601
Malhotra K, Baer M, Li YF, Sancar GB, Sancar A. 1992. Identification of chromophore
binding domains of yeast DNA photolyase.
J. Biol. Chem. 267:2909–14
Malhotra K, Kim S-T, Batschauer A, Dawut L,
Sancar A. 1995. Putative blue-light photoreceptors from Arabidopsis thaliana and
Sinapis alba with a high degree of sequence
homology to DNA photolyase contain the
two photolyase cofactors but lack DNA repair activity. Biochemistry 34:6892–99
Malhotra K, Kim S-T, Sancar A. 1994. Characterization of a medium wavelength type
DNA photolyase: purification and properties
of photolyase from Bacillus firmus. Biochemistry 33:8712–18
Masamoto K, Kinoshita T, Shimizaki K. 1993.
Light-induced de-epoxidation of violaxanthin in guard cell protoplasts of Vicia faba.
Plant Cell Physiol. 34:935–38
Matsui M, Stoop CA, von Arnim AG, Wei N,
Deng X-W. 1995. Arabidopsis COP1 protein specifically interacts in vitro with a
cytoskeleton-associated protein CIP1. Proc.
Natl. Acad. Sci. USA 92:4239–43
McNellis TW, Torii KU, Deng X-W. 1996. Expression of an N-terminal fragment of COP1
confers a dominant-negative effect on lightregulated seedling development in Arabidopsis. Plant Cell 8:1491–503
McNellis TW, von Arnim AG, Deng X-W.
1994. Overexpression of Arabidopsis COP1
results in partial suppression of lightmediated development: evidence for a lightinactivable repressor of photomorphogenesis. Plant Cell 6:1391–400
Meijer G. 1968. Rapid growth inhibition of
gherkin hypocotyls in blue light. Acta Bot.
Néerl. 17:9–14
Millar AJ, Short SR, Chua N-H, Kay SA. 1992.
A novel circadian phenotype based on firefly luciferase expression in transgenic plants.
Plant Cell 4:1075–87
Millar AJ, Straume M, Chory J, Chua N-H, Kay
SA. 1995. The regulation of circadian period
by phototransduction pathways in Arabidopsis. Science 267:1163–66
Miyamoto Y, Sancar A. 1998. Vitamin B2-based
blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments
for setting the circadian clock in mammals.
Proc. Natl. Acad. Sci. USA 95:6097–102
Mockler TC, Guo H, Yang H, Duong H, Lin C.
1999. Antagonistic action of the Arabidopsis cryptochromes and phytochrome B in the
regulation of floral induction. Development.
In press
Mohr H. 1994. Coaction between pigment systems. In Photomorphogenesis in Plants, ed.
RE Kendrick, GHM Kronenberg, pp. 353–
73. Dordrecht, Netherlands: Kluwer. 2nd Ed.
Neff MM, Chory J. 1998. Genetic interactions between phytochrome A, phytochrome
?
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 118:27–
36
Ninnemann H. 1997. Cooperative blue/UVlight absorbing pigments in fungal and plant
photoreception. In Modern Topics in Photochemistry and Photobiology, ed. F Vargas,
pp. 115–54. Trivandrum, India: Research
Signpost
Niyogi KK, Grossman AR, Björkman O. 1998.
Arabidopsis mutants define a central role for
the xanthophyll cycle in the regulation of
photosynthetic energy conversion. Plant Cell
10:1121–34
Noh B, Spalding EP. 1998. Anion channels and
the stimulation of anthocyanin accumulation
by blue light in Arabidopsis seedlings. Plant
Physiol. 116:503–9
Nozue K, Kanegae T, Imaizumi T, Fukada S,
Okamoto H, et al. 1998. A phytochrome from
the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl. Acad.
Sci USA 95:15826–30
Osterlund MT, Deng X-W. 1998. Multiple
photoreceptors mediate the light-induced
reduction of GUS-COP from Arabidopsis
hypocotyl nuclei. Plant J. 16:201–8
Palmer JM, Warpeha KMF, Briggs WR. 1996.
Evidence that zeaxanthin is not the photoreceptor for phototropism in maize coleoptiles.
Plant Physiol. 110:1323–28
Pang Q, Hays JB. 1991. UV-B-inducible and
temperature-sensitive photoreactivation of
cyclobutane pyrimidine dimers in Arabidopsis thaliana. Plant Physiol. 95:536–43
Parks BM, Cho MH, Spalding EP. 1998. Two
genetically separable phases of growth inhibition induced by blue light in Arabidopsis
seedlings. Plant Physiol. 118:609–15
Parks BM, Quail PH, Hangarter RP. 1996.
Phytochrome A regulates red-light induction
of phototropic enhancement in Arabidopsis.
Plant Physiol. 110:155–62
Pellequer J-L, Wager-Smith KA, Kay SA,
Getzoff ED. 1998. Photoactive yellow protein: a structural prototype for the threedimensional fold of the PAS domain super-
59
family. Proc. Natl. Acad. Sci. USA 95:5884–
90
Poppe C, Schäfer E. 1997. Seed germination
of Arabidopsis thaliana phyA/phyB double
mutants is under phytochrome control. Plant
Physiol. 114:1487–92
Poppe C, Sweere U, Drumm-Herrel H, Schäfer
E. 1998. The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana.
Plant J. 16:465–71
Quail PH. 1991. Phytochrome: a light-activated
molecular switch that regulates plant gene expression. Annu. Rev. Genet. 25:389–409
Quail PH. 1997. The phytochromes: a biochemical mechanism of signaling in sight?
BioEssays 19:571–79
Quail PH, Boylan MT, Parks BM, Short TW, Xu
Y, et al. 1995. Phytochromes: photosensory
perception and signal transduction. Science
268:675–80
Quail PH, Briggs WR, Chory J, Hangarter RP,
Harberd NP, et al. 1994. Spotlight on phytochrome nomenclature. Plant Cell 6:468–
71
Quiñones MA, Lu Z, Zeiger E. 1996. Close correspondence between the action spectra for
the blue light responses of the guard cell and
coleoptile chloroplasts, and the spectra for
blue light-dependent stomatal opening and
coleoptile phototropism. Proc. Natl. Acad.
Sci. USA 93:2224–28
Quiñones MA, Zeiger E. 1994. A putative role
of the xanthophyll, zeaxanthin, in blue light
photoreception of corn coleoptiles. Science
264:558–61
Rebbapragada A, Johnson MS, Harding GP,
Zuccarelli AJ, Fletcher HM, et al. 1997. The
Aer protein and the serine chemoreceptor Tsr
independently sense intracellular energy levels and transduce oxygen, redox, and energy
signals for Escherichia coli behavior. Proc.
Natl. Acad. Sci. USA 94:10541–46
Reymond P, Short TW, Briggs WR. 1992. Blue
light activates a specific protein kinase in
higher plants. Plant Physiol. 100:655–61
Rüdiger W, Briggs Wr. 1995. Involvement of
?
P1: FHN/fgi
P2: FHN
September 20, 1999
60
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
thiol groups in blue-light-induced phosphorylation of a plasma membrane-associated
protein from coleoptile tips of Zea mays L.
Z. Naturforsch. 50c:231–34
Sachs (von) J. 1887. Lectures on the Physiology
of Plants, p. 696. Oxford: Clarendon. (Eng.
Ed. HM Ward translator)
Salomon M, Zacherl M, Rüdiger W. 1997a.
Phototropism and protein phosphorylation in
higher plants: unilateral blue light irradiation generates a directional gradient of protein phosphorylation across the oat coleoptile. Bot. Acta 110:214–16
Salomon M, Zacherl M, Rüdiger W. 1997b.
Asymmetric blue light-dependent phosphorylation of a 116-kilodalton plasma membrane protein can be correlated with the firstand second-positive phototropic curvature of
oat coleoptile. Plant Physiol. 115:485–91
Sancar A. 1994. Structure and function of DNA
photolyase. Biochemistry 33:2–9
Sancar GB, Jorns MS, Payne G, Fluke DJ, Rupert CS, et al. 1987. Action mechanism of
Escherichia coli DNA photolyase III. Photolysis of the enzyme-substrate complex and
the absolute action spectrum. J. Biol. Chem.
262:492–98
Senger H, ed. 1980. The Blue Light Syndrome.
Berlin: Springer-Verlag.
Senger H, ed. 1984. Blue Light Effects in Biological Systems. Berlin: Springer-Verlag.
Senger H, ed. 1987. Blue Light Responses: Phenomena and Occurrence in Plants and Microorganisms. Boca Raton, FL: CRC
Senger H, Briggs WR. 1981. The blue light receptor(s): primary reactions and subsequent
metabolic changes. In Photochemical and
Photobiological Reviews, ed KC Smith, 6:1–
38. New York: Plenum
Shand RF, Betlach MC. 1991. Expression of
the bop gene cluster of Halobacterium halobium is induced by low oxygen tension and
by light. J. Bacteriol. 173:4692–99
Short TW, Briggs WR. 1994. The transduction
of blue light signals in higher plants. Annu.
Rev. Plant Physiol. Plant Mol. Biol. 45:143–
71
Shropshire W Jr. 1980. Carotenoids as primary
photoreceptors in blue-light responses. See
Senger 1980, pp. 172–86
Small GD, Min B, Lefebvre PA. 1995. Characterization of a Chlamydomonas reinhardtii
gene encoding a protein of the DNA
photolyase/blue light photoreceptor family.
Plant Mol. Biol. 28:443–54
Söderbäck E, Reyes-Ramirez F, Eydmann T,
Austin S, Hill S, et al. 1998. The redoxand fixed nitrogen-responsive regulatory protein NIFL from Azotobacter vinelandii comprises discrete flavin and nucleotide-binding
domains. Mol. Microbiol. 28:179–92
Somers DE, Devlin PF, Kay SA. 1998. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282:1488–90
Spalding EP, Cosgrove DJ. 1988. Large plasmamembrane depolarization precedes rapid
blue-light-induced growth inhibition in cucumber. Planta 178:407–10
Spalding EP, Cosgrove DJ. 1992. Mechanism of
blue-light-induced plasma-membrane depolarization in etiolated cucumber hypocotyls.
Planta 188:199–205
Srivastiva A, Zeiger E. 1995a. Guard cell zeaxanthin tracks photosynthetically active radiation and stomatal apertures in Vicia faba
leaves. Plant Cell Environ. 18:813–17
Srivastiva A, Zeiger E. 1995b. The inhibitor of
zeaxanthin formation, dithiothreitol, inhibits
blue light-stimulated stomatal opening in Vicia faba. Planta 196:445–49
Stanewsky R, Kaneko M, Emery P, Beretta B,
Wagner-Smith K, et al. 1998. The cryb mutation identifies cryptochrome as a circadian
photoreceptor in Drosophila. Cell 95:681–
92
Stowe-Evans EL, Harper RM, Motchoulski AV,
Liscum E. 1998. NPH4, a conditional modulator of auxin-dependent differential growth
responses in Arabidopsis. Plant Physiol. 118:
1265–75
Tagliatela M, Castaldo P, Iossa S, Pannaccione
A, Fresi A, et al. 1997. Regulation of the human ether-a-gogo related gene (HERG) K+
?
P1: FHN/fgi
P2: FHN
September 20, 1999
15:2
Annual Reviews
AR092-02
BLUE-LIGHT PHOTORECEPTORS
channels by reactive oxygen species. Proc.
Natl. Acad. Sci. USA 94:11698–703
Teramura A. 1996. How plants respond to a
changing UV-B radiation environment. In
Regulation of Plant Growth and Development by Light, ed. WR Briggs, RL Heath,
EM Tobin, pp. 164–70. Rockville, MD: Am.
Soc. Plant Physiol.
Thresher RJ, Vitaterna MH, Miyamoto Y,
Kazantsev A, Hsu DS, et al. 1998. Role
of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science
282:1490–94
Todo T, Ryo H, Yamamoto K, Toh H, Inui T,
et al. 1996. Similarity among the Drosophila
(6-4)photolyase, a human photolyase homolog, and the DNA photolyase-blue light
photoreceptor family. Science 272:109–
12
Travis AJ, Mansfield TA. 1981. Light saturation of stomatal opening on the adaxial and
abaxial epidermis of Commelina communis.
J. Exp. Bot. 32:1169–79
van der Spek PJ, Kobayashi K, Bootsma D,
Takao M, Eker AP, et al. 1996. Cloning,
tissue expression, and mapping of a human photolyase homolog with similarity to
plant blue-light receptors. Genomics 37:177–
82
Vince-Prue D. 1994. The duration of light and
photoperiodic responses. In Photomorphogenesis in Plants, ed. RE Kendrick, GHM
Kronenberg, pp. 447–90. Dordrecht, Netherlands: Kluwer. 2nd ed.
Wada M, Sugai M. 1994. Photobiology of ferns.
In Photomorphogenesis in Plants, ed. RE
Kendrick, GHM Kronenberg, pp. 783–802.
Dordrecht, Netherlands: Kluwer. 2nd Ed.
Wald G, du Buy HG. 1936. Pigments of the oat
coleoptile. Science 84:247
Wang X, Iino M. 1997. Blue-light-induced
shrinking of protoplasts from maize coleoptiles and its relationship to coleoptile growth.
Plant Physiol. 114:1009–20
Wang X, Iino M. 1998. Interaction of cryptochrome 1, phytochrome, and ion fluxes in
blue-light-induced shrinking of Arabidopsis
61
hypocotyl protoplasts. Plant Physiol. 117:
1265–79
Warpeha KMF, Kaufman L. 1989. Blue-light
regulation of epicotyl elongation in Pisum
sativum. Plant Physiol. 89:544–48
Wei N, Kwok SF, von Arnim AG, Lee A, McNellis TW, et al. 1994. Arabidopsis COP8,
COP10, and COP11 genes are involved in repression of photomorphogenic development
in darkness. Plant Cell 6:629–43
Whitelam GC, Devlin PF. 1998. Light signalling in Arabidopsis. Plant Physiol. Biochem. 36:125–33
Yamamoto HY, Kamite L. 1972. The effects of
dithiothreitol on violaxanthin de-epoxidation
and absorbance changes in the 500 nm region. Biochim. Biophys. Acta 267:538–42
Yeh K-C, Lagarias JC. 1998. Eukaryotic phytochromes: light-regulated serine/threonine
protein kinases with histidine kinase ancestry. Proc. Natl. Acad. Sci. USA 95:13976–
81
Young JC, Liscum E, Hangarter RP. 1992.
Spectral-dependence of the light-inhibited
hypocotyl elongation in photomorphogenic
mutants of Arabidopsis: evidence for a UVA photosensor. Planta 188:106–14
Zacherl M, Huala E, Rüdiger W, Briggs WR,
Salomon M. 1998. Isolation and characterization of cDNAs from oat encoding a
serine/threonine kinase: an early component in signal transduction for phototropism
(Accession Nos. AF033096 and AF033097)
(PGR98–028). Plant Physiol. 116:869
Zeiger E, Helpler P. 1977. Light and stomatal function: blue light stimulates swelling
of guard cell protoplasts. Science 196:887–
89
Zeiger E, Zhu J. 1998a. Sensory transduction of
blue light in guard cells. Plant Biology ‘98,
Program and Suppl., p 9. Abstr. 11006
Zeiger E, Zhu J. 1998b. Role of zeaxanthin in
blue light photoreception and the modulation
of light-CO2 interactions in guard cells. J.
Exp. Bot. 49:433–42
Zhao S, Sancar A. 1997. Human blue-light photoreceptor hCRY2 specifically interacts with
?
P1: FHN/fgi
P2: FHN
September 20, 1999
62
15:2
BRIGGS
■
Annual Reviews
AR092-02
HUALA
protein serine/threonine phosphatase 5 and
modulates its activity. Photochem. Photobiol. 66:727–31
Zhong HH, Resnick AS, Straume M, McClung
CR. 1997. Effects of synergistic signaling by
phytochrome A and cryptochrome 1 on cir-
cadian clock-regulated catalase expression.
Plant Cell 9:947–55
Zhulin IB, Taylor BL. 1997. PAS domain Sboxes in archaea, bacteria and sensors for
oxygen and redox. Trends Biochem. Sci. 22:
331–33
?