Testicular Cancer - American Urological Association
advertisement

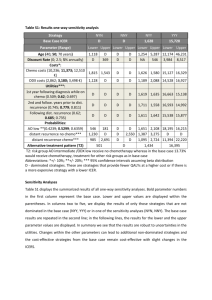
Testicular cancer Badrinath R. Konety, MD Classification • • • • • • Germ Cell Tumors (GCTs) Gonadal Stromal tumors Tumors with GCT + Stromal elements Hematopoeitic/Lymphoid Tumors of Rete Testis Adnexal tumors GCT - Introduction • Most common in men 15-35 yrs • Paradigm for multimodality treatment • Survival < 50% (1970) to > 95% (1997) • Focus on decreased morbidity GCT - Epidemiology • Around 7000 new cases in USA • 90-95% of all testicular primary neoplasms • Most common solid tumors of men age 20 to 34 years • 2-3 % bilateral • Cryptorchidism 3-14 times ↑ risk 5-10% contralateral orchiopexy helps detection • Trauma • Atrophy • Hormones (mothers on estrogens) Classification GCTs Seminoma Classic Anaplastic Spermatocytic Non Seminoma Embryonal carcinoma Yolk Sac Choriocarcinoma Teratoma Mixed NSGCTs Clinical Manifestations • • • • Painless testis swelling/lump Acute pain (10%) Reactive hydrocele Metastatic disease - 40-50% pts – Respiratory / GI / Back pain / DVT etc • Gynecomastia – 5% • Physical Exam - Testicular self exam • Scrotal Ultrasonography Seminoma • • • • Enlarged painless testis 35-70% of all GCTs. PLAP + in 90% Can be bilateral either synchronous or metachronous Classic 85%, age 30-50 syncitiotrophoblasts (15%) = b HCG • Anaplastic 5-10% more aggressive, 1/3rd of all deaths from seminoma, more bHCG • Spermatocytic 2-10%, no metastasis, >40yrs. Most common GCT in men > 65 yrs 10% of metastatic seminoma has NSGCT elements Campbell-Walsh Urology, Elsevier, Philadelphia, PA NSGCTs • • • • • Embryonal Choriocarcinoma Teratoma Yolk Sac Mixed Embryonal Carcinoma • 40% of all GCTs have EC elements • Usually < 2 cm, VI, LI adverse prognosis • Syncitiotrophoblastic cells adjacent to EC cells, produce b HCG • If AFP ↑, usually from coexistent yolk sac • EC cells do not secrete either AFP or bHCG • Age 25-35 yrs • Hard, irregular testis, normal size Teratoma • < 5% in adults, 40% in children • By definition, 2 or more germ cell layers • Endo – mucus secreting • Meso – Bone cartilage, muscle • Ecto – squamous, neural • AFP in 25% (mucinous or hepatoid differentiation) Mature teratoma with cartilege Teratoma • Most often seen 10-30y • Benign in children • Can undergo malignant transformation in adults to sarcoma, squamous carcinoma or adenocarcinoma • Resistant to chemoradiation • Metastatic teratoma – most often embryonal Immature teratoma Choriocarcinoma • 1-2%, pure or mixed • Pure CC – hemorrhagic mass with viable tumor cells at periphery • Syncitiotrophoblasts + cytotrophoblasts • Necrosis and hemorrhage • B HCG > 99% • Decades 2 and 3 • No testis swelling, small nodule • Usually present with distant mets – lung and /or brain • Hematogenous mets most common Mixed GCTs • 60% of all GCTs • Even if seminoma present, treat as NSGCT • Serum tumor markers may or may not be elevated Yolk Sac tumor (Embryonal Carcinoma of infants and children ) Most common prepubertal testis tumor AFP ↑ >90% of pts Hydrocele in 25% pts Pattern of mets different in children (more hematogenous spread) • Confined to testicle in 80% of cases • Low volume RP disease - RPLND • • • • Intratubular Germ Cell Neoplasia (ITGCN) • • • • • • Adjacent to germ cell tumor: 98% Cryptorchidism: 2-8% Prior germ cell tumor (contralateral testis): 5% 50% risk of developing GCT in 5y Treat with observation, XRT (20 Gy) or orchiectomy Chemo reduces risk but still 25% - 45% risk of GCT at 10y (Christensen et al. Ann. Oncol. 1998) • Can be precursor to all types of GCT except spermatocytic seminoma GCT – Unique Features • Chemo and radiation • Retroperitoneum is usually sensitive the first and only site of • Capacity to differentiate metastatic disease • Consistent pattern of • Landing zones for rightmetastasis sided tumors: – Interaortocaval, precaval • Ability to produce marker lymph nodes substances (AFP/HCG) • NSGCT: unique potential for • Landing zones for left-sided tumors: teratomatous – Para-aortic, left hilar differentiation lymph nodes • High growth rates 10-30 days Right to Left Crossover extremely common Clinical Staging TNMS • Findings at Inguinal Orchiectomy – Histology, size, extent of invasion, LVI • Imaging – Chest and Retroperitoneum – CT scan with IV contrast – PET CT more accurate for seminoma rather than NSCGT • Serum Tumor Markers – AFP, bHCG , LDH NSCGT • Choriocarcinoma – can have brain mets • Brain mets can bleed during chemo • Check head CT particularly in patient with very high bHCG and choriocarcinoma Serum markers • AFP ↑ in 50% , b HCG ↑ in 50% with NSGCT • 90% of NSGCT have either one marker ↑ • 10-15% will have neg markers even in advanced disease • Normalization of markers after treatment ≠ absence of disease in 10-20% pts AFP - AlphaFetoProtein • Half-life of 5-7 days • Elevated in embryonal, yolk-sac, mixed • Excludes diagnosis of seminoma/pure choriocarcinoma • False positive with liver disease (hepatitis, cirrhosis) and drug abuse (alcohol, cocaine) • Can be elevated in hepatocellular, pancreatic, stomach, and lung cancers b HCG- Human Chorionic Gonadotrophin • Half Life of 24-36 hours • Assay cross reactive with β subunit of LH (↑in castration du to high GnRH) • Also ↑ in liver, pancreas, stomach breast, lung cancers and marijuana smokers • ↑ in chorio/embyronal/ seminoma (10%) LDH • • • • Produced by liver, muscle >200 U/dl Can be elevated in various types of GCT Good marker for seminoma Testes sparing surgery • • • • • • Controversial Mass <2cm Simultaneous bilateral tumors Solitary testicle with normal testosterone Biopsy adjacent parenchyma (80% ITGCN) Can treat remaining testicle with 20Gy of XRT Scrotal violation • Local recurrence higher (2.9% vs 0.4%) (Capelouto et al. J. Urol. 1995) • Seminoma – extend radiation portal to include groin and scrotum area • NSCGT – excise scar and cord remnant • Extensive groin resection or hemiscrotectomy not required especially after chemo NSCGT stage I • 20-30% occult RP mets • LVI and Embryonal predominance (45-90% ) are risk factors • Absence of yolk sac and MIB-1 + staining >70% ↑ risk • ↑ rate of mets from 35-70% • Surveillance – – – – 28% relapse and 1.4% mortality 90% relapses <2y, most in RP Chemo if large mass and ↑ markers RPLND if nl markers and small mass NSCGT stage I – RPLND • • • • • • Full bilateral template <2% local recurrence Curative in 60-90% with IIa Needs adjuvant chemo for >IIa 100% curative for teratoma Lower recurrence free survival compared to primary chemo 92% vs 99% (Albers et al. JCO 2008) Stage I NSCGT - chemo • • • • • • 2 cycles of BEP Best single modality cure rate Does not treat teratoma Long term surveillance Late toxicity Under treats >IIb disease Guidelines: Low risk stage I – surveillance High risk stage I – surveillance/chemo/RPLND Stage IIa-b • RPLND – – – – Upto 1/3 neg nodes Treats teratoma Only 2 cycles chemo Avoids chemo in upto 50% • Chemo – 60-80% avoid surgery – Treatment in community – CSS >95% Stage IIc and III NSCGT • Risk based chemo – Good risk • BEP x 3 or EP x 4 – Intermediate and Poor risk • BEP x 4 • BEP x 3 = EP x 4 (94% vs 91% 5y RFS Culine et al. An Oncol 2007) Risk Criteria Good Test/RP primary + no non pulm visceral mets + AFP <1000, bHCG <5000, LDH <1.5x normal Intermediate Test/RP primary + no non-pulm visceral mets + AFP 1000-10,000 OR bHCG 5000-50,000 OR LDH 1.5x to 10x normal Poor Mediastinal primary OR non-pulm visceral mets OR AFP >10,000 OR bHCG >50,000 OR LDH >10x normal PC- RPLND • Indicated mass >1cm negative markers • Lower relapse after PC RPLND • Better response to second line chemo • <1cm mass, no teratoma in primary and good risk disease – observe CSS >96% (Ehrlich et al. JCO 2010) Path Frequency Fibrosis 40% Teratoma 45% Tumor 15% PC relapse • Prognostic characterstics – >10% of specimen with tumor – Intermediate or poor risk GCT – Incomplete resection • Accepted salvage regimens add 4 cycles – Vinblastine, Ifosfamide,Platinum (VIP), – Paclitaxel, Ifosfamide, Platinum (TIP), – Vinblastine, Etoposide, Ifosfamide, Platinum (VeIP) • High dose appears to be = std dose • Growing teratoma – stop and resect PC masses • Post second line chemo mass – – – – 53% viable tumor; 21% teratoma No benefit of post RPLND chemo Complete resection less feasible Desperation surgery (RPLND only) • Late relapse >2y (3%) – 54-88% tumor (yolk sac most common) – 12-28% teratoma – 10% malignant transformation (adenoca most common) – Poor response to second line chemo Seminoma- Clinical Stage I options 5 year survival > 95% Adjuvant XRT 20-30 Gy (preferred in US) Paraaortic / Hockey stick field Primary chemotherapy (1-2 cycles carboplatin) Long term toxicity, recurrence rate 3-5% • Treat Clinical stage I S with primary chemo Active Surveillance – low risk (<4cm, no LVI) Rigorous follow up/compliance/ imaging related radiation exposure 17% RELAPSE at 15 months, OS similar Seminoma risk factors • • • • • Size (> or < 4cm) Rete testes invasion LVI – not important No poor risk category High risk stage I – 2 cycles vs 1 cycle chemo (Aparicio J et al. JCO 2011) Risk Criteria Good risk Any primary + no non pulm visceral mets + normal markers Intermediate risk Any primary + presence of non pulmonary visceral mets + normal markers Seminoma treatment Stage treatment Stage IIa XRT 35Gy Stage IIb XRT or Chemotherapy (EP x 4 or BEP x 3) Stage IIc Risk based chemotherapy (EP x 4 or BEP x3) Stage III Risk based chemotherapy (EP x 4 or BEP x 3) RP/pelvic recurrence after dog leg XRT <1% - no need for follow up CT RP recurrence after chemo – same (need CT f/u) 3-5% relapse after 1 cycle carboplatin Seminoma post chemo mass • • • • • 60-80% will have residual masses Majority will resolve over 12-18 months 90% necrosis and 10% viable tumor Mass <3cm – observe Mass >3cm – evaluate with PET – If PET positive then RPLND (very difficult) Seminoma - relapse • Surveillance failures – dog leg XRT • Bulky adenopathy - chemotherapy Toxicity of chemo/XRT Early Late Very late Fatigue Raynauds Secondary malignancies Myelosuppression Neuropathy CV disease Neuropathy Hearing loss GI disease Hearing loss Hypogonadism Infection Infertility Mortality (upto 4.4%) RPLND Templates Avoid dissection below IMA on contralateral side Reducing Extra-Template Residual Disease • Inclusion of all infrahilar regions except the contralateral iliac would reduce residual postRPLND disease to: 2% for right-sided templates 3% for left-sided templates • 3-32% can have disease outside templates Eggener et al, J Urol, 2007, Carver et al. JCO 2007 RPLND: Technique Matters • Paracolic recurrences occur following incomplete excision of the spermatic cord • Re-operative RPLND has a higher morbidity rate and adversely impacts survival • Nerve-sparing is essential to achieve antegrade ejaculation – Sympathetic chain – Paravertebral sympathetic ganglia – Post-gangionic fibers T2-L4 Kantzavelos, Urology, 2003 Donohue et al, Semin Urol Oncol, 1998 Chang, J Urol, 2002 McKiernan, Urology, 2003 What about RPLND following chemotherapy for metastatic disease? • Full bilateral RPLND is currently the standard of care • Several groups have recommended omitting PC-RPLND in men with a clinical complete response to chemotherapy • Modified templates have been suggested in the post-chemotherapy setting with limited supporting data • Redo RPLND associated with worse survival (55% vs 84%) (Donahue et al. Sem. Urol. Oncol. 1998) Ehrlich, BJU, 2006 Donohue, Urol Oncol, 2003 Permpongkosol, Urology, 2007 Post-chemo residual mass Small post-chemo mass Daneshmand et al. Urol. Oncol. 2011 Limited Post-Chemotherapy Resections are Associated with Decreased Disease Specific Survival All patients Fibrosis Overall Complete RPLND (n=459) Complete RPLND (n=459) Complete RPLND (n=227) Resection of Mass (n=20) Resection of Mass (n=45) Resection of Mass (n=45) p<0.001 P<0.001 Carver et al, JCO 2007 p=0.002 Complications • Remember all complications !!! • Injury to any intraabdominal structures – Bowel (duodenum), pancreas • vasculature (renal, SMA, IMA etc…), cisterna chylli – chylous ascites • Ureter • Perioperative complications related to prior chemotherapy • Ejaculatory dysfunction Avoid complications • Careful fluid resuscitation in post chemo patients • Low inspired O2 • Spinal cord ischemia due to aortic mobilization (1%) • Ileus/bowel obstruction • Pancreatitis Non GCT tumors • Leydig cell tumor – 25% occur in kids – Present with painless mass and precocious puberty – In adults feminizing characteristics – 90% benign 10% malignant (almost all in adults) – Orchiectomy is treatment – Mets poor response to XRT/chemo (Mitotane) • Sertoli cell Non GCT – 1% or tumors 90% benign – Orchiectomy and RPLND if mets • Granulosa cell – Benign, orchiectomy • Gonadoblastoma – – – – Intersex and mixed gonadal dysgenesis Female with amenorrhea or cryptorchid male GCT elements can metastasize Bilateral orchiectomy Hematologic malignancies • Lymphoma – Most common in men >60y age – Could be primary or recurrent – Orchiectomy • Leukemia – Testes sanctuary site – Common in children – Bilateral in 50% – Rx with chemo/XRT A recently married, 25-year-old man, had a radical orchiectomy for embryonal cell carcinoma. There was lymphatic invasion in the rete testis. Serum markers, chest and abdominal CT scans are normal. His semen analysis shows the volume of 3 ML, 25 million sperm per ML, motility 60%, and 50% normal forms. The patient and his wife want the treatment that gives the best chance for fertility, as well as the best cancer control. He should be advised to undergo A. B. C. D. E. Radiotherapy Surveillance Modified retroperitoneal lymphadenectomy Bilateral retroperitoneal lymphadenectomy Platinum based combination chemotherapy A 24-year-old man is treated for stage III mixed germ cell tumor of the right testis by inguinal orchiectomy, four cycles of BEP chemotherapy, and post-chemotherapy bilateral RPLND. He remains disease-free with normal serum tumor markers for four years. Current physical examination and serum HCG are normal. Serum AFP is 27 mg/dl (normal less than 10 mg/dl), and a CT scan of the chest, abdomen, and pelvis demonstrates a solitary 2 cm mass in the right retrocrural area. The next step is A. B. C. D. E. Observation Repeat AFP after IM testosterone. Additional BEP chemotherapy Salvage chemotherapy Excision of the mass A 27-year-old man has bulky retroperitoneal adenopathy following radical orchiectomy for a mixed germ cell tumor. His chest x-ray is normal. Serum beta-HCG and AFP are markedly elevated. Liver enzymes are slightly elevated and the patient relates a history of ethanol excess. He receives three cycles of BEP. Restaging reveals a 3 cm retroperitoneal mass, a normal chest x-ray, and normal serum beta-HCG. However, the serum AFP is 20 IU/ML (normal equals 0-9). The next step is A. B. C. D. E. Retroperitoneal lymph node dissection. CT-guided needle biopsy Salvage chemotherapy Serial markers and CT scans External beam radiotherapy A 26 -year-old man undergoes orchiectomy for seminoma. A CT scan of the retroperitoneum and tumor markers are negative. He elects surveillance. The most accurate statement regarding his outcome over the next 2 years is: A. 5% risk of visceral relapse B. Similar to patients treated with radiation therapy C. 40% risk of relapse with nonseminomatous elements D. 5% risk of retroperitoneal node relapse E. 50% risk of pulmonary relapse Modified RPLND preserves fertility in most patients by sparing which of the following structures? A. B. C. D. E. Internal iliac arteries. Genitofemoral nerve. Postganglionic sympathetic nerve fibers. Seminal vesicles. Pelvic parasympathetic plexus. For a 2.5 cm residual retroperitoneal mass post chemotherapy for advanced pure seminoma (pretreatment retroperitoneal mass 8 cm in diameter) the optimal next step in management is: A. Consolidation radiotherapy. B. Second time chemotherapy. C. Excision of the residual mass. D. Surveillance. E. Autologous marrow transplant and further chemotherapy. A 64 -year-old man has painless right testicular swelling of 3 months duration. Urinalysis is normal, and testicular ultrasound reveals an enlarged right testis. The most likely diagnosis is: A. B. C. D. E. Lymphoma. Chronic lymphocytic leukemia. Spermatocystic seminoma. Teratocarcinoma. Embryonal carcinoma.