Cabbage pH Indicator Lab
advertisement

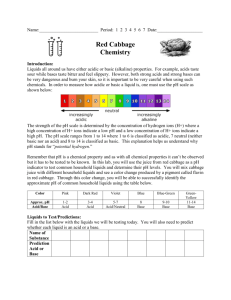
Rosie Research and the Color Changing Cabbage One cold, dark morning Rosie Research was jetting out of her house. “Late as usual” Rosie thought as she grabbed her breakfast. This morning it was a breakfast bar and a glass of her power packed purple smoothie. Rosie watered it down with a bottle on the table and raced out the door to catch her bus. When she finally sat down she pulled out her breakfast and was shocked to see her smoothie wasn’t purple. “That’s odd” thought Rosie, “I’m positive it was purple this morning in the fridge” She looked down into the perfectly pink drink in her hands. What was going on? She sipped it. “Yuck!” Rosie exclaimed. This was not her usual power smoothie. This pink drink was absolutely disgusting. Rosie thought back. Was she imagining thigns? It was purple this morning right? It was definitely purple in the fridge. Right? What would have made it pink? And why did it taste so different now? www.RosieResearch.com Let’s help Rosie find out! First, let’s make her smoothie. You can make purple cabbage juice up to a week in advance and store it in the fridge or freezer! 1. Cut up one red cabbage 2. Boil it until the water turns dark purple (30-45 minutes) 3. Toss the cabbage and keep the juice! 4. When you are ready you will need a pencil, crayons, cabbage juice and anything under the sun to test. Now that we have Rosie’s smoothie, let’s do what she did. Let’s add some water. Did that turn it pink? Color in the glasses before and after you add in the water. What do you think Rosie added? Write in your guess and test it. Red Cabbage Juice Red Cabbage Juice + water Then find some other household items and test them as well usingthe right hand sheet. Wow! Red Cabbage Juice + ____________ Check around your house and find some liquids, gels, powders or even pills of interest. Write in what you added to each cup and color them in depending on what color the cabbage juice turns. Then use our color meter to determine if it is an acid or a base! Need some ideas? Orange juice, lemons, apples, baking soda, cream of tartar, soap, conditioner, heartburn medicine, hand sanitizer...once you get started you won’t want to stop! © 2015 www.RosieResearch.com © Name Color pH value (from chart) Notes Sort your findings acording to their color into acidic and basic categories Strongly Acidic Weakly Acidic Neutral Weakly Basic © 2015 www.RosieResearch.com © Strongly Basic Name Color pH value (from chart) Notes Name Color pH value (from chart) Notes Mixing it up! Is there a way to turn Rosie’s smoothie back to purple? What would you add? Would it taste the same? + ___________ + ___________ Red Cabbage Juice Make your own chain reactions. It’s fun adding one thing at a time to purple cabbage juice, but what about adding to the mix? Make your own chains of reaction and then try to figure out how to navigate through our puzzles. + + + + ___________ ___________ ___________ ___________ Red Cabbage Juice Red Cabbage Juice + ___________ + ___________ + ___________ + ___________ Red Cabbage Juice + ___________ + ___________ + ___________ + ___________ Let’s solve the mystery! What did Rosie Research add to her morning smoothie? After sorting various foods and juices into acids and bases, what can you determine about how acids taste? What about bases? What would have Rosie’s drink tasted like? How does it work? Red cabbage juice is purple because of anthocyanin, a pigment often found in flowers, fruits and leaves. In fact, it is the cause of many of the reds, blues purples and oranges we see around us everywhere. Colors of the world around us are determined by how molecules like anthocyanin absorb light. They can absorb light into their outer shell electrons (called valence electrons). The energy absorbed into the bonds through light is light that will never reach our eyes, so if red light is absorbed, a substance would look blue. When we add in acid we change the structure of anthosyanin. That is because adding acid is adding in a bunch of hydrogen ions (H+), and oxygen can bind with that hydrogen. Can you see the difference between the acidic molecule below and the one above? Adding base is like adding a bunch of hydroxide ions (OH-). This also changes the molecule. Can you spot the difference? All of these changes to the molecule changes the bonds. With different bonds we absorb different energies, and thus different wavelengths, or colors, of light! Keep on Sleuthing. Flavinoids, Flavinoids, Everywhere. The molecule anthocyanin, a flavinoid, is found in the coloring of fruist, vegetables and even leaves all around us. Blackberries, blueberries, cherries, plums, red apples, wines and even red beets all are chalk full of flavinoids. Experiment with other plants that could be used as a pH indicator like cabbage. Which one works the best? Not so secret anymore. Red cabbage juice will only change color where a strong acid or strong base are present. Using full strength lemon juice write a secret message on a piece of paper. After it dries use red cabbage juice to paint over the paper. What do you see? Homemade pH paper. You can make your own pH paper, also known as litmus paper, using red cabbage juice and coffee filters. Just soak the filters, dry, and cut into strips. Now you can take your pH testing out of the lab and into the wild! Upload your projects! We would love to see your science sleuthing in action. Upload your photos and notes by emailing us at info@rosieresearch.com PreK-2