transporters
advertisement

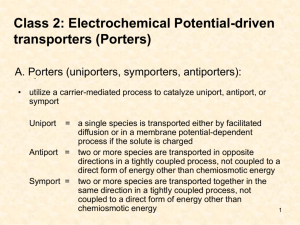
Secondary active transport use the ion gradients established by ATPase for transport of various substances against their gradients of electrochemical potentials via transporters/ carriers. Transporters tend to be highly specific for their substrates, therefore the cell has many diverse transporters in the membrane. 1 The carriers/porters form complexes by binding their solutes before transporting them across the bilayer by the secondary processes of symport or antiport. They are also called electrical potential-driven transporters. Class of carriers a very large family is called the major facilitator superfamily (MFS). ionophores, nonribosomally synthesized carriers (e.g. valinomycin and nigericin) Ton B, family of proteins involved in transferring energy to the outer membrane of Gram-negative bacteria. Luckey, M. 2008. Membrane Structural Biology. 2 Major Facilitator Superfamily (MFS) Lactose permease (LacY) and the glycerol-3-phosphate transporter (GlpT) of E. coli are members of the MFS of secondary active transporters. LacY, a galactoside/H+ symporter, utilizes the proton gradients of the inner membrane to drive the 100-fold accumulation of lactose inside cell. GlpT, an organophosphate/phosphate antiporter, takes up glycerol-3phosphate for use as an energy source and as a precursor for phospholipids. X-ray structure of LacY Luckey, M. 2008. Membrane Structural Biology. 3 GlpT. Model of conformational changes proposed to accompany substrate translocation (rocker-switch transporters) The protein alternates between an outward-facing conformation (Co) and inward-facing conformation (Ci). The substrate phosphate (red) is shown as it moves from the inside (C) to the outside (A). The crystal structure corresponds to the inward-facing conformation without substrate (D). The outward-facing conformation was generated by rotating the two halves of GlpT by 16° each in opposite direction (B), while the Co-Pi conformation (A) required a 10° rotation. Luckey, M. 2008. Membrane Structural Biology. The common architecture, and likely mechanism, of MFS transporters does not entirely reveal how these uniporters, symporters, and antiporters for widely varied substrates carry out secondary active transport. 5 Leucine transporter Members of the solute carrier 6 (SLC6) family of sodium-coupled transporters, also known as neurotransmitter sodium symporters, make up one of the most widely investigated and pharmacologically important classes. SLC6 proteins play a central role in diverse physiological processes, ranging from the maintenance of cellular osmotic pressure to the reuptake of small-molecule neurotransmitters in the brain. SLC6 dysfunction is implicated in numerous debilitating illnesses, such as depression (8), obsessive-compulsive disorder, epilepsy, autism, orthostatic intolerance, X-linked creatinedeficiency syndrome, and retinal degeneration. The transport activity of these molecular machines can be inhibited by many different compounds, including tricyclic antidepressants (TCAs), selective serotonergic reuptake inhibitors, anticonvulsants, and cocaine. LeuT occluded-state structures. Superposition of the LeuT-Leu (gray), LeuT-Ala (green), LeuT-Gly (magenta), LeuT-Met (blue), and L-4-F-Phe (orange) complexes by use of α-carbon positions. Membrane boundaries are demarcated by the two solid black lines. Singh et al.. 2008. Sci. 322:1655. Unraveling the molecular principles that define a substrate (a molecule that can be transported) versus a competitive inhibitor (a molecule that can displace the substrate but is not itself transported) is intimately linked to the larger goal of elucidating transport mechanism and ultimately to the development of new therapeutic agents. The leucine transporter (LeuT), a prokaryotic SLC6 member (18), provides an opportunity to couple functional and structural data to uncover the molecular mechanisms of transport and inhibition. 6 Amino acid transporters (ApcT) Water Amino acid, polyamine, and organocation (APC) transporters are members of a large family of secondary transport proteins that catalyze the uniport, symport, and antiport of a broad range of substrates across the membrane bilayer. ApcT is a broad-specificity amino acid transporter. Architecture of ApcT. (A) Ribbon diagram of the ApcT structure, viewed parallel to the membrane, along the pseudo 2-fold axis of molecular symmetry. (B) "Top" down view of ApcT from the outside. (C) Slice through a solvent-accessible surface of ApcT showing a solvent-accessible pathway reaching deep into the transporter. Water molecules are shown as cyan spheres. (D) Superposition of the scaffold helices TMs 3 to 5 and 8 to 10 of ApcT and vSGLT onto the equivalent elements of LeuT shows that in ApcT, TM1b is closed to the outside and TM1a is partially open to the inside. TMs 2 to 10 of LeuT are shown as an α-carbon trace. Shaffer. 2009. Sci. 325:1010. 7 Mitochondrial ADP/ATP Carrier Mitochondria are surrounded by two membranes: an outer membrane with pores that allow fairly nonselective passage of molecules and ions up to around 500 Da, and an inner membrane with at least 20 specific transport functions. The ADP/ATP carrier (AAC) provides the ADP substrate needed inside the matrix for ATP synthase and to export the ATP it produces. The AAC is an electrogenic antiporter that exchange one ADP3- for one ATP4- without bound Mg2+ ions, with the net export of one negative charge driven by the membrane potential. Indispensable to the generation of ATP by respiration, AAC is the most abundant of the mitochondrial carriers and makes up 10% of the protein extracted from the inner mitochondrial membrane. 8 Facilitated diffusion through ion transporters Ion transporters are differentiated from channels by their much slower rate of turnover, by thermodynamic differences (transporters have one infinite energy barrier in contrast to finite barriers at all times in channels) and by their primary structure. 9 Transporters/Carriers exhibit saturation kinetics (Vmax). Permeation of ions involves binding and de-binding of ions to a specific site, together with conformation changes of the proteins. (A) (B) Km is Michaelis-Menten constant 10 Turnover rates • Pump long-range and complex conformation transitions • Transporter/ Carrier conformation changes, but no long-range interaction with soluble substrates • Channel no conformation changes during transport. Channels have the greatest turnover rate of all enzymes. 11 Buchanan et al. 2000. Turnover rates Transport system Molecule transported per second 2 Pumps 10 Transporters 2 10 -10 5 6 8 Channels 10 -10 12 Buchanan et al. 2000. Transport proteins in plant plasma membrane Pumps Channels Transporters 13 Overview of the various transport processes on the plasma membrane and the tonoplast of plant cells Cytosol pH = 7.2 ∆E = - 120 mV = Ecytosol- Eextracellular Vacuole pH = 5.5 ∆E = - 90 mV = Evacuole- Eextracellular 14 Taiz and Zeiger. 2006. Overview of the various transport processes on the tonoplast Electrical potential, mV pH∼5.5 pH 7.0-7.4 การปั๊ ม H+ ผ่าน plasma membrane ออกจาก เซลล์ ทําให ้ pH ภาย นอกเซลล์ตํา่ กว่าใน cytosol และการปั๊ ม H+ ผ่าน tonoplast เข ้าสู่ vacuole ทําให ้ pH ของ vacuole มีคา่ ตํา่ กว่าใน cytosol ในทํานองเดียวกัน ั ย์ไฟฟ้ าของ cytosol ศก ตํา่ กว่าภายนอกประมาณ 120 mV และตํา่ กว่า vacuole 10-30 mV กล่าวคือ pH ของ cytosol มีคา่ สูงกว่าอีก สองบริเวณ และมี ั ย์ไฟฟ้ าตํา่ สุด ศก Cytosol pH∼5.5 16 Thylakoid (internal) membrane STROMA (low H+) LUMEN (high H+) 17 Taiz and Zeiger. 2006. Mitochondria Transmembrane transport in plant mitochondria. An electrochemical proton gradient consisting of a membrnae potential (∆E=-200mV, negative inside) and a ∆pH (alkaline inside) is established across the inner mitochondrial membrane during electron transport. 18 Taiz and Zeiger. 2006. Mitochondria 19 Taiz and Zeiger. 2006. Confocal mapping of cellular pH via fluorescence intensity ratio imaging. Rengel. 2002. 20