Pocket Redox
advertisement

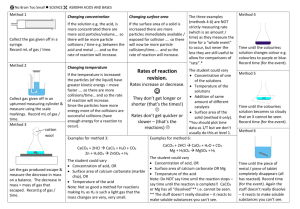
POCKET REVISION THE SCIENCE SCRIBE LV II CHEMISTRY: REDOX BY LIAN SOH INSTRUCTIONS: These can be found at: www.sciencescribe.co.nz RIGHTS: Students, teachers and schools are granted permission to photocopy/print multiple copies of this document. Sale of this document without written permission from the author is prohibited. If uncertain, contact info@sciencescribe.co.nz 1 CHLORINE Cl2 Pale green gas/solution Bubbles of chlorine gas are… 2 CHLORIDE - Cl Clear colourless Sodium chloride solution is… …bubbles were observed. 3 BROMINE Br2 Red liquid or orange solution …mixed with bromine water. …orange decolourises. 4 BROMIDE - Br Clear colourless Potassium bromide solution is… …orange colour forms 5 NITRIC ACID (DIL.) + H Clear colourless solution …added to dilute nitric acid. …bubbles observed. 6 HYDROGEN H2 Clear colourless gas Bubbles of hydrogen gas are … …litmus turns red. 7 IRON (III) IONS 3+ Fe Orange/yellow solution …added to iron sulfate solution. …orange turns green. 8 IRON (II) IONS 2+ Fe Pale green solution Iron (II) sulfate solution is… …green turns pale yellow. 9 COPPER (II) IONS 2+ Cu Blue solution. …added to copper sulfate solution. …blue disappears, shiny orange/pink solid forms. 10 COPPER Cu Shiny orange/pink solid. Copper metal is… …metal gets smaller; reaction gains a blue tint. 11 HYPOCHLORITE - OCl Clear colourless …added to bleach. 12 (REV. ON 2) CHLORIDE Cl - Clear colourless Potassium chloride solution is… …bubbles observed. 13 IODINE I2 Grey purple (s). Brown (aq). …added to iodine solution. …brown decolourises. 14 IODIDE I - Clear colourless Sodium iodide is… …grey solid forms; light brown tint to solution also observed. 15 OXYGEN O2 Clear colourless gas …reacted with oxygen. 16 OXIDE 2- O Clear colourless Choose a different left-sided page. 17 HYDROGEN PEROXIDE H2O2 Clear colourless liquid ...added to hydrogen peroxide. 18 WATER H2O Clear colourless liquid Choose a different left-sided page. 19 DICHROMATE 2- Cr2O7 Orange solution …mixed with potassium dichromate. …orange turns green. 20 CHROMIUM (III) 3+ Cr Green/blue solution. Choose a different left-sided page. 21 PERMANGANATE - MnO4 Purple solution …mixed with potassium permanganate. …purple decolourises. 22 MANGANESE (II) Mn 2+ Colourless solution Choose a different left-sided page. 23 NITRIC ACID (CONC.) - NO3 Clear colourless solution …mixed with concentrated nitric acid. …brown gas evolves. 24 NITROGEN DIOXIDE NO2 Brown gas. Choose a different left-sided page. 25 IODATE - IO3 Clear colourless solution …mixed with sodium iodate solution. (REV. ON 14) IODIDE 26 I - Clear colourless solution Sodium iodide is… …grey solid forms; light brown tint to solution also observed. 27 SULFUR S Yellow solid Choose a different right-sided page. 28 HYDROGEN SULFIDE H2S Clear colourless gas Gaseous hydrogen sulfide is… …yellow solid forms. 29 SULFATE 2- SO4 Clear colourless Choose a different right-sided page. 30 SULFUR DIOXIDE SO2 Clear colourless gas Gaseous sulphur dioxide is… 31 SULFATE 2- SO4 Clear colourless Choose a different right-sided page. 32 SULFITE 2- SO3 Clear colourless solution Sodium sulfite is… 33 SULFATE 2- SO4 Clear colourless Choose a different right-sided page. 34 HYDROGEN SULFITE - HSO3 Clear colourless solution Sodium hydrogen sulfite is… 35 OXYGEN O2 Clear colourless liquid Choose a different right-sided page. 36 HYDROGEN PEROXIDE H2O2 Clear colourless liquid Hydrogen peroxide is… …effervescence was observed 37 (REV. ON 8) IRON (III) 3+ Fe Orange/yellow solution reacted with iron (III) chloride solution. …yellow turned solution turns pale green. 38 IRON Fe Grey shiny solid A nail is… …nail gained an orange hint. 39 MAGNESIUM (II) Mg 2+ Clear colourless solution reacted with magnesium nitrate solution. …grey solid particles form. 40 MAGNESIUM Mg Shiny grey solid Magnesium metal is… …metal gets smaller. OXIDATION NUMBERS (ANS) PAGE 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) 11) 12) 13) 14) 15) 16) 0 -1 0 -1 +1 0 +3 +2 +2 0 +1 -1 0 -1 0 -2 PAGE PAGE 17) 18) 19) 20) 21) 22) 23) 24) 25) 26) 27) 28) 29) 30) 31) 32) 33) 34) 35) 36) 37) 38) 39) 40) -1 -2 +6 +3 +7 +2 +5 +4 +5 -1 0 -2 +6 +4 +6 +4 +6 +4 0 -1 +3 0 +2 0 ©Lian Soh 2013