Experiment 13
advertisement

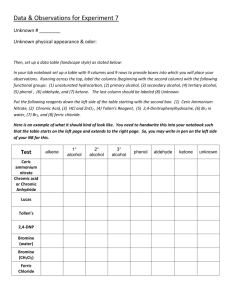
Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College Experiment N. Qualitative Identification of Organic Compounds by Functional Group Differentiation 1. LAB ASSIGNMENT: Study G&M classification tests found in Chapter 25, Identifying Organic Compounds: ceric nitrate (Sec. 25.12, p. 868); 2,4-DNP (Sec. 25.7, p. 841); chromic acid (Sec. 25.7, p. 845); iodoform (Sec. 25.7, p. 848); Tollens' silver mirror (Sec. 25.7, p. 844); bromine in dichloromethane (Sec.25.8, p. 851); silver nitrate (Sec. 25.9, p. 854). 2. EXPERIMENTAL OBJECTIVES: The objective of this experiment is to use qualitative classification tests to identify letter-coded samples of known organic compounds. Given a list of six (6) compounds, all clear and colorless liquids, and samples (labeled A-F) of all six, identify the unknowns by functional group differentiation all of the samples. 3. POINT VALUE OF LAB: Question Outline : 20 points. Laboratory Report: 30 points. 4. SPECIAL INSTRUCTIONS: a. Work in groups of two, submit one laboratory report on the enclosed form, due at the end of the laboratory period. b. Each member of your team must complete the Question Outline (problems 1-4 of this document) before the laboratory period and submit it for grade at the beginning of the laboratory period. Your team will not be permitted to proceed through the analyses without both teammates having completed the Question Outline. c. Chemicals and solvents: 1) pentanal, [110-62-3] 2) tert-butylchloride (2-chloro-2-methylpropane), [507-20-0] 3) 1-butanol, [71-36-3] 4) 1-hexene, [592-41-6] 5) 2-propanol, [67-63-0] Page 1 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College 6) 2-propanone, [67-64-1] 7) Ceric Nitrate Test: ceric ammonium nitrate [16774-21-3], 1,4-dioxane [123-91-1] 8) 2,4-DNP Test: 2,4-dinitrophenylhydrazine [119-26-6], sulfuric acid [7664-93-9], ethanol, [64-17-5] 9) Chromic Acid Test: chromic anhydride (CrO3) [1333-82-0], sulfuric acid [7664-93-9], acetone [67-64-1] 10) Iodoform Test: iodine [7553-56-2], KI [7681-11-0], dioxane [123-91-1] 11) Tollen’s Test: silver nitrate [7761-88-8], KOH [1310-58-3], NH4OH [7664-41-7], nitric acid [7697-37-2] 12) Bromine Decolorization Test: bromine [7726-95-6] in dichloromethane [75-09-2] 13) Silver Nitrate Test: silver nitrate [7761-88-8], ethanol [64-17-5], nitric acid [7697-37-2] d. Reagent recipes. All recipes are scalable. Reagents have already been prepared for students. 1) Ceric Nitrate Reagent. Add 1.3 mL concentrated nitric acid to 40 mL water. Then dissolve 10.96 g ceric ammonium nitrate in the solution. Dilute the solution to 50 mL. 2) 2,4-DNP Reagent. 0.2 g 2,4-DNP in 1 mL concentrated sulfuric acid, then add 1.5 mL water and then 5 mL 95% ethanol. 3) Chromic Acid Reagent. 1 g CrO3 to 1 mL concentrated sulfuric acid, stir to make paste. Dilute paste with 3 mL water. 4) Iodoform Reagent. Make solution of 2 g KI and 8 mL water. Then add 1 g I2. 5) Tollen’s Test Reagents. Reagent A: 0.25 g silver nitrate in 4.3 mL water. Reagent B: 0.3 g KOH in 4.2 mL water. 6) Bromine in Dichloromethane. Make 0.1 M solution of bromine in dichloromethane. 7) Silver Nitrate Reagent. Make a solution of 0.5 g silver nitrate in 30 mL 95% ethanol. Page 2 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College Experiment N. Question Outline and Procedures. 1. The series of reactions you will perform to identify the samples involve a qualitative test for a particular functional group or molecular fragment. A classification test gives an immediate “yes” or “no” to the question, “Does this unknown contain the functional group or fragment for which this reagent tests?” The combination of several tests will allow you to determine, for example, that compound X contains a carbonyl moiety, the carbonyl is an aldehyde, and the alkyl group adjacent to the carbonyl is a methyl group. 2. You have studied several of the reactions in class. Some are new and can be found in the laboratory textbook. Knowing what constitutes a positive test, in the form of a color change or precipitation, will enable you to quickly test the samples and collect the data you will need to establish a means of solving these identification problems. 3. The following table shows the structures of all of the compounds you will identify. Name the compound and the functional group and/or moiety present in the molecule. OH OH Compound name: Functional group present: O O Cl H Compound name: Functional group present: Page 3 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College 4. Now that you know what unknowns you must identify, familiarize yourself with the tests that will give you information about what functional groups are present. There are seven chemical tests you will perform on the unknowns. Knowing what reaction is occurring and what physical change constitutes a positive test result will enable you to quickly progress through the analyses. Complete the following table using G&M or the optional references as noted in the Advance Sheet. Use the format provided in the table entry for the ceric nitrate classification test for alcohols as you complete the remainder of the table. Some entries are provided for you. Classification Test (text, page ref) Reaction 1 ceric nitrate test for alcohols (NH4)2Ce(NO3)6 + RCH2OH → (NH4)2Ce(OCH2R)(NO3)5 + HNO3 2 2,4-DNP test for Indicator of positive test yellow → red; no precipitate Test limitations Aliphatic amines → white ppt blue-to-green opaque suspension Look for color change in 2 s. 3° ROH, ketones give (-) tests unless adulterated ________________________________? 3 chromic acid test for 1°, 2° ROH and aldehydes RCH2OH or CrO3 R(C=O)H H O+ 3 Page 4 of 10 RCOOH + H2O + Cr2(SO4)3 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) 4 School of Science and Technology Georgia Gwinnett College iodoform test for ________________________________? 5 silver mirror (Tollen’s) test for ________________________________? (note limitation for 1° ROH) Page 5 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) Classification Test (text, page ref) 6 School of Science and Technology Georgia Gwinnett College Reaction bromine in dichloromethane test for Indicator of positive test Test limitations Electrophilic aromatic subst. with arenes ________________________________? 7 silver nitrate test in ethanol for ________________________________? Page 6 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College 5. Procedures for the classification tests. a. Concept. The classification tests must be performed in a sequence that will allow you to pare down the number of samples for subsequent tests. Specifically, conduct both the ceric nitrate and 2,4-DNP tests on all six samples. Based on the results of these two tests, subject only the samples that tested [+] for either of these two tests to the next two tests (chromic acid and iodoform). Then test only samples which tested [+] for DNP with the Tollen’s silver mirror test. Finally, analyze only the samples yielding [─] results for both the ceric nitrate and 2,4-DNP tests by the last two tests (Br2 in CH2Cl2 and AgNO3 in ethanol). b. Classification test procedures. Use 5 mL test tubes for all tests. Quantities are estimated; consider the conversion of 1 mL ~ 20 drops of liquid volume. Unknown = “unk”. The qualitative results you observe within the first few seconds are the valid ones. Do not change the results you record after the solutions stand for a few minutes; these are not valid. test # test Qty/vol reagent #1 qty/vol reagent #2 qty/vol reagent #3 1 ceric nitrate 8–10 drops (NH4)Ce(NO3)6 3–4 drops unk color change in 5–10 sec 2 2,4-DNP 3 drops unk 6–8 drops 2,4-DNP solution yellow to red ppt 3 chromic acid 2 drops unk 10 drops acetone 4 iodoform test 2 drops unk + 5 drops 3M NaOH(aq);; Δ to 60º 1mL KI-I2 soln→brown 5 6 Tollen’s silver mirror Br2 in CH2Cl2 6 drops Tollen’s A solution + 1 drop Tollen’s B solution (see ppt of Ag2O) 2 drops unk 7 AgNO3 in EtOH 5 drops 0.1M AgNO3(aq) in 95% EtOH 2 drops NH4OH (conc) (Ag2O dissolves) 2 - 3 drops soln of 2% Br2 in CH2Cl2 2 drops unk dropwise addn of CrO3/H3O+ dropwise addn of up to 1 ml of 10% KOH→clear 1 drop unk specific instructions blue-green ppt in 2 sec yellow ppt in clear soln is[+] black ppt brown color disappears/fades ppt appears in < 5 sec c. Waste disposal. Empty the contents of the test tubes into the waste bottle in the disposal hood. Rinse the test tubes with acetone, then add the rinse to the waste at the disposal hood. Throw the empty test tubes into the broken glass container. Page 7 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College 6. Classification Tests Results—Summary. Complete this table during the laboratory periods. Include your observations and hypotheses. Insert + for positive test results; ─ for negative test results; and N/A for test not performed. Perform in sequence: Test → Cmpd ↓ Α 1. ceric nitrate test all samples 2. 2,4-DNP test all samples 3. chromic acid 4. iodoform test only samples that give [+] for either ceric nitrate or DNP 5. Tollen’s silver mirror test samples that give [+] for DNP only Β C D E F Page 8 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College 6. Classification Tests Results—Summary (cont.). Test 6. Br2 in CH2Cl2 7. AgNO3 in ethanol → Cmpd↓ test only the samples that gave [-] for both ceric nitrate and DNP A B C D E F When you have recorded all of the data for the qualitative tests, present this record to your professor. Professors’ initials:__________ Page 9 of 10 6/20/08 Organic Chemistry II Lab CHEM 2212L (Summer 2008) School of Science and Technology Georgia Gwinnett College 7. Identification of samples. Complete the following table identifying the unknowns. Place the letter OH A-F in the block next to the structure. Give a brief justification for your choice; e.g., [+] for carbonyl by 2,4-DNP; [+] for aldehyde by Tollen’s; [+] for methyl ketone by iodoform; O therefore acetaldehyde Cl OH O H Page 10 of 10 6/20/08