full version - Stony Brook University
advertisement
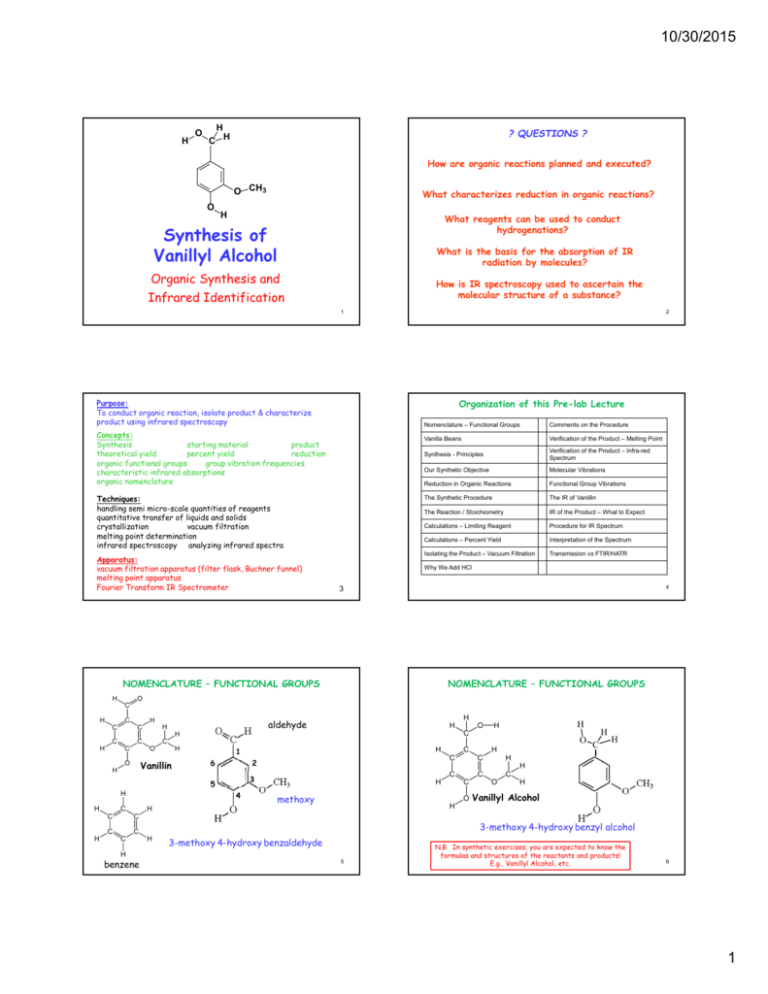
10/30/2015 H O H C H ? QUESTIONS ? How are organic reactions planned and executed? O CH3 O What characterizes reduction in organic reactions? H What reagents can be used to conduct hydrogenations? Synthesis of Vanillyl Alcohol What is the basis for the absorption of IR radiation by molecules? Organic Synthesis and Infrared Identification How is IR spectroscopy used to ascertain the molecular structure of a substance? 1 Organization of this Pre-lab Lecture Purpose: To conduct organic reaction, isolate product & characterize product using infrared spectroscopy Concepts: Synthesis starting material product theoretical yield percent yield reduction organic functional groups group vibration frequencies characteristic infrared absorptions organic nomenclature Techniques: handling semi micro-scale quantities of reagents quantitative transfer of liquids and solids crystallization vacuum filtration melting point determination infrared spectroscopy analyzing infrared spectra Apparatus: vacuum filtration apparatus (filter flask, Buchner funnel) melting point apparatus Fourier Transform IR Spectrometer 2 Nomenclature – Functional Groups Comments on the Procedure Vanilla Beans Verification of the Product – Melting Point Synthesis - Principles Verification of the Product – Infra-red Spectrum Our Synthetic Objective Molecular Vibrations Reduction in Organic Reactions Functional Group Vibrations The Synthetic Procedure The IR of Vanillin The Reaction / Stoichiometry IR of the Product – What to Expect Calculations – Limiting Reagent Procedure for IR Spectrum Calculations – Percent Yield Interpretation of the Spectrum Isolating the Product – Vacuum Filtration Transmission vs FTIR/HATR Why We Add HCl 4 3 NOMENCLATURE – FUNCTIONAL GROUPS H H H C C H C C C C C H O H C H Vanillin O aldehyde H H H C C C 6 C C H H 2 3 4 C H 1 5 H NOMENCLATURE – FUNCTIONAL GROUPS O H methoxy C H C C C O O H H O C C H O H C H C H H H Vanillyl Alcohol 3-methoxy 4-hydroxy benzyl alcohol H 3-methoxy 4-hydroxy benzaldehyde H benzene C H 5 N.B. In synthetic exercises, you are expected to know the formulas and structures of the reactants and products! E.g., Vanillyl Alcohol, etc. 6 1 10/30/2015 MAJOR CONSTITUENTS OF THE RIPE VANILLA BEAN SYNTHESIS Conducting one or more chemical REACTIONS using STARTING MATERIALS to produce a desired PRODUCT What makes a synthesis EFFECTIVE ? H C O If it produces the desired product: • from reasonable STARTING MATERIALS • under practical CONDITIONS e.g., accessible temperature & pressure • at a reasonable RATE (CATALYSTS may be helpful) • with relatively high YIELD (Few by-products) CH2OH OCH3 OCH 3 OH OH vanillyl alcohol vanillin 7 8 OUR OBJECTIVE • in a chemical environment that permits practical SEPARATION OF PRODUCT from excess starting materials and byproducts To synthesize: • in an ENVIRONMENTALLY RESPONSIBLE way - GREEN CHEMISTRY • in a CO$T-EFFECTIVE manner For substances that may be consumed by humans or animals, there is an additional concern: + H2 SYNTHESIS must not involve TOXIC AGENTS – either chemical or biological - that are NOT EASILY REMOVED How do you decide what starting materials to use? What readily available substance has a related structure? What reactions would accomplish the change in structure? 9 10 REDUCTION In organic chemistry, reduction often means addition of a hydrogen molecule to a multiple (e.g., double) bond. H-H + H H C C H H ethylene C C H H H H ethane C O H H formaldehyde + 3 H2 Ni H C O C=O H + 3 H2 Pd methyl alcohol Hydrogen can be added to organic compounds in many ways. As hydrogen gas, H2: REDUCTION USING H2 H H H H H-H + Vanillyl Alcohol Vanillin 11 CH4 + H2O H2 will reduce the C≈C bonds in the benzene ring in vanillin and the C=O bond to CH4. 12 2 10/30/2015 ARE THERE OTHER WAYS TO ADD H2? SODIUM BOROHYDRIDE – NaBH4 Different ways of adding hydrogen give different results depending on type of multiple bonds in reactant. We seek a way to add two hydrogen atoms (i.e., reduce) to the C C==O O bond ≈ C without reducing C bonds in benzene ring. H Na+ H Hydride Ion H- B H H A reagent which accomplishes this is: 13 BH4- is isoelectronic with CH4 and NH4+ SYNTHETIC PROCEDURE STOICHIOMETRY OF THE REACTION You will be handling small quantities of materials. 400 mg of vanillin (C8H8O3) - [ 2.6 mmol ] 2.5 mL of 1.0 M NaOH - O 400 ± 40 mg but exactly The reagents 2.5 ± 0.2 mL [ 2.5 mmol ] 80 ± 8 mg but exactly C H O H 4 H C H 4 OCH3 80 mg of sodium borohydride (NaBH4)- [ 2.1 mmol ] After reaction is complete, 14 OCH3 OH + BH4- + 4 H2O OH + H3BO3 + OH- Less than 10 mL of 2.5 M HCl - [ 25 mmol ] Must exercise care in transferring such small amounts between containers. 15 16 CALCULATIONS – LIMITING REAGENT CALCULATIONS – PERCENT YIELD 100 X Actual yield Pct yield = ----------------------Theoretical yield 100 X Actual yield Pct yield = ----------------------Theoretical yield Theoretical yield = maximum yield that could be produced from actual amount of limiting reagent. E.g., 0.4120 g vanillin (MM = 152) 412 mg / 152 = 2.71 mmol E.g., 0.0825 g NaBH4 (MM = 38) 82.5 mg / 38 = 2.2 mmol Theoretical yield = maximum yield that could be produced from actual amount of limiting reagent. Limiting Reagent E.g., 0.4120 g vanillin (MM = 152) Could make 2.71 mmol vanillyl alcohol But, stoichiometry is 1 NaBH4 ↔ 4 Vanillin so, we could reduce 4 X 2.2 mmol = 8.8 mmol Vanillin 17 412 mg / 152 = 2.71 mmol 2.71 X 154 = 417 mg If you actually recover 349 mg 100 X 349 % Yield = ---------------- = 83.7% 417 18 3 10/30/2015 ISOLATING THE PRODUCT WHY WE ADD HCl The vanillyl alcohol product is isolated from the reaction mixture by crystallization from water and vacuum filtration (See web supplement.) What is in the reaction vessel after the reaction? We assume the most insoluble component is the product and that its solubility is lowest at low temperatures (ice bath). In addition, • we dissolved the vanillin in NaOH and • OH- was also produced in the reaction Vanillin is the limiting reagent so some NaBH4 remains unreacted. But, H3BO3 can react with NaOH to form Na3BO3 Vanillyl alcohol decomposes in either strongly acid OR basic solutions. HCl is added to decompose BH4- & make solution neutral. BH4- (and OH-) react with HCl (aq) http://www.ic.sunysb.edu/Class/che133/techniques/suctfilt/suctfilt.html BH4- + 3H2O + H+ → H3BO3 + 4 H2 (g) 19 PROCEDURE – SOME NOTES 20 VERIFYING SYNTHETIC SUCCESS – 1 THE MELTING POINT Pay close attention to directions: • Add NaBH4 slowly to cold solution Melting points are a simple way to obtain evidence in support of the identity and purity of a substance. (See the web supplement). • Let reaction mixture stand at room temperature They are determined using the following apparatus: • Chill Solution to 0oC For 30 min • Chill with ice for recommended period For 10 min • Add HCl, adjusting pH to acid litmus test slowly. Be sure that entire solution is just acidic (pink), but not excessively. You must dry a small amount (½ spatulaful) of the sample for melting point and IR. 21 http://www.ic.sunysb.edu/Class/che133/techniques/meltpts/meltpts.html MOLECULAR VIBRATIONS VERIFYING SYNTHETIC SUCCESS - IR The structures of the starting and product molecules in this exercise (and many syntheses) differ in a way that makes infrared spectroscopy an appropriate analytical tool to establish identity of the product (and a rough indication of its purity) Have previously done a virtual lab on absorption spectroscopy of food dyes (ultraviolet and visible). 0.9 0.8 where, 1/μ = 1/m1 + 1/m2 k is a the strength of the spring (bond) 0.7 0.6 0.5 Some Typical Infra-red Frequencies 0.4 0.3 0.2 0.1 Wavelength (nm) Absorption of light in infrared region is primarily due to VIBRATION of molecules. (1 μm = ) 1,000 nm – 100,000 nm (Infra-red) 23 740 71 0 68 0 65 0 62 0 590 56 0 53 0 50 0 47 0 44 0 41 0 380 0 35 0 Absorbance The frequency of molecular vibrations depends on the mass of the atoms involved and the strength of the bonds between them. For two atoms, the relationship is: To involve numbers in a convenient range, it is customary to report f/c where c = velocity of light in cm/sec. Since f = c, this just 1/, which we measure in cm-1. Absorbance vs Wavelength 1 Those absorptions were due to transitions between ELECTRONIC energy levels (UV) 350 nm – 700 nm (Red) 22 Molecule f/c (cm-1) H-H 4161 H-D 3632 H-F 3961 H-I Molecule f/c (cm-1) Molecule f/c (cm-1) H-Cl 2885 N-O 2220 H-Br 2558 C-O 2143 2230 Cl-Cl 557 24 4 10/30/2015 WHAT ABOUT POLYATOMIC MOLECULES? GROUP VIBRATIONS Study of many thousands of substances shows that SPECIFIC MOLECULAR FRAGMENTS absorb light at well-defined, characteristic frequencies, e.g., H2O 3835 cm-1 3939 cm-1 1648 cm-1 Symmetric Stretch Bend Asymmetric Stretch f (cm-1) CH4 3025 cm-1 1367 cm-1 3157 cm-1 C — H (aromatic) 3030 – 3050 C — H (alkane) 2850 – 2960 C == O (aldehyde) 1680 – 1750 H H C H C C C C O O — H (phenol) O — H (alcohol) C O ~1100 ~1650 ~1720 C H ~2900 25 Table 2 of SUPL-005 shows the absorption frequencies in cm-1 of some molecular fragments “Aromatic” means Here are some that are related to benzene or benzene-like today’s exercise. 1600, 1500 C C 1367 cm-1 http://www2.ess.ucla.edu/~schauble/molecular_vibrations.htm C C (aromatic) C C 3200 26 VANILLIN IR SPECTRUM: 500 cm-1 – 4000 cm-1 O H H H C O C H C H 3400 – 3650 27 Note that like visible spectra, IR spectra are displayed as intensity vs increasing wavelength http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng 28 VANILLIN IR SPECTRUM: 1500 cm-1 – 4000 cm-1 BUT as Percent Transmittance (instead of absorbance), and indicating the (decreasing) wavenumber scale instead of wavelength % Transmittance So, absorption peaks point DOWNWARD O-H -H Wavelength etc. 29 4000 cm-1 3000 cm-1 C-H3 HC=O 2000 cm-1 CC 30 1500 cm-1 5 10/30/2015 H H cm O -1 C VANILLIN IR SPECTRUM: 1500 cm-1 – 4000 H C H H C H C O C C O C H H H H C O C H C H C C C O H C H H H H H C H C O CC C H IR OF THE PRODUCT H H H H C O C H C H C H C C C C C O H O H H O H H C H H What other difference should there be? There should be a new absorption due to O−H in alcohol group. That absorption is near, but distinct from, the O—H absorption due to the OH group on the ring (phenol At ~3200 cm-1). ring hydrogen stretch C−H stretch in the methoxy (O-CH3)group C−H3 C O So, the product spectrum should show the absence of the C=O absorption near 1700 cm-1. -O−H stretch due to the OH group on the ring HC=O H C C O H The vanillin spectrum shows 6 major absorption peaks between 1500 and 4000 cm-1. −H C C C=O stretch in the aldehyde group From the table we see that we expect it at: two peaks due to the ring CC stretch All but one of these peaks should show up in the spectrum of the product, vanillyl alcohol. 31 H2C O—H between 3400 and 3600 cm-1 in the alcohol O-H region. 32 IR Spectrometer IR SPECTRUM OF VANILLYL ALCOHOL When sample is DRY, • obtain the spectrum of a small sample using the FTIR Spectrometer. Follow the posted instructions •Analyze the spectrum to identify the peaks due to the product (and, if any, due to the starting material) You may wish to examine the IR spectrum of pure vanillyl alcohol before coming to lab. You can download it from: The ZnSe sample area http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng 33 INTERPRETATION OF IR SPECTRA Use the infrared spectrum to verify the presence or absence of functional groups Reaction replaces a -HC=O group by a –H2C-O-H. So, while starting material will show: absorption by -HC=O no absorption by –H2C-O-H Product should show: absorption by –H2C-O-H no absorption by –HC=O a new O-H absorption Should also be able to identify absorptions of other functional groups common to vanillin and vanillyl alcohol by 35 comparing their spectra. 34 TRANSMISSION vs FTIR/HATR SPECTROSCOPY UV-visible spectrometer: FTIR: Scans individual wavelength – measures %T at that wavelength - proceeds to next wavelength, etc. Scans all wavelengths at once measures total %T – changes source intensity profile at high rate and measures total %T as a function of time. FTIR: Fourier Transform Infra Red Transmission Spectroscopy: Reflection Spectroscopy: I0 () I0 (t) It () Ir (t) HATR: Horizontal Attenuated Total Reflectance 36 6






