Antidepressants
advertisement

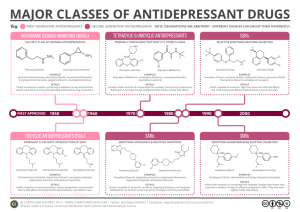
Antidepressants Morten Grøtli Department of Chemistry and Molecular Biology Email: (grotli@chem.gu.se) Useful reading (refer to Foye’s 7th ed) • Chaper 18 , page 570 Neurotransmitter deficiency syndroms and their interactions Monoamine hypothesis Serotonin nurotransmission Norephinephrine nurotransmission Types of antidepressive drugs Antidepressive drugs Monoamine reuptake inhibitors Selective Nonselective Monoamine oxidase inhibitors Miscellaneous Selectivity ratios for reuptake inhibitors Norephinephrine reuptake Serotonin reuptake TCA Tricyclic antidepressants TCAs Introduced in 1957 by isosteric replacement of phenothiazines’ “S” with a “C-C” isostere This isosteric replacement also causes a change in the numbering system Numbering begins at the first carbon next to the isosteric replacement General structure If there is a substituent on one of the rings, then start numbering on that ring Tricyclic antidepressants Tricyclic antidepressants α is decreased, a β angle and γ angle is introduced causing the ring to twist dramatic decrease in affinity for the dopamine receptor; most TCAs are no longer dopamine antagonists, while a few have weak dopamine antagonism Increased affinity for the norepinephrine, serotonin presynaptic membrane transporter (reuptake) TCA’s work by competitively inhibiting reuptake for norepinephrine and serotonin TCA’s are generally more selective for the norepinephrine transporter as compared to serotonin with the sole exception of clomipramine Tricyclic antidepressants Tricyclic antidepressants Features common to all TCAs include: (1) A protonatable nitrogen, (2) Two aromatic rings and (3) Approximately a 2-carbon distance between the protonatable nitrogen and an aromatic ring. TCA Substitutions Not much ring substitution seen with these drugs, unsubstituted phenyl rings are equal. Removal of a phenyl ring results in total loss of activity Electron withdrawing groups are not important for activity as with the phenothiazines Electron withdrawing groups can change selectivity from norepinephrine transporter to serotonin transporter and some substitutions can result in a loss of activity (Clomipramine) The C10–C11 bridge can be an ethylene or vinyl with no change in activity. In fact the C10–C11 bridge is not required; breaking the ring at this point can retain activity (see SSRIs) γ Nitrogen Substituents Tricyclic antidepressants 1. Potency is in order: 3º > 2º > 1º amine; 2. Although overall the TCAs are selective for the norepinephrine transporter (except clomipramine) the 3 amine is more selective for the serotonin transporter as compared to the corresponding 2° amine; and 2 amines are more selective for NE transporter; 3. 3° amines are more anticholinergic and antihistaminergic than 2° amines. In fact more bulk on the nitrogen follows the anticholinergic SAR; 4. Ethyl or higher alkyl groups lead to a loss of activity and an increase in toxicities. Toxicity increases with increasing chain length; 5. 2o amines’ antidepressant activity followed by stimulation. Tricyclic antidepressants 1. Based on the middle ring, or ring B Dibenzazepines: have a clomipramine, desipramine) nitrogen at C5 (Imipramine, trimepramine, Dibenzepines: no heteroatoms (Amitriptyline, nortryptiline, protryptiline) Other tricyclics: some have an O in the epine ring, or an O and an N, an N in the eleven position, or a 6 membered middle ring (Doxepine) Tricyclic antidepressants Based on the substitution of the aliphatic nitrogen a) 2o amines: differences as discussed before (Desipramine, nortryptiline, protryptiline) b) 3o amines: differences as discussed before (Imipramine, trimipramine, clomipramine, amitryptyline, doxepine) Common TCA side effects include Adrenergic, Seretonergic, Anticholinergic, Antihistaminic, α Blocker, Quinidine-like effect What angles are present here? Metabolism of clomipramine Tricyclic antidepressants Maprotiline: The ethyl bridge forms a fourth ring (tetracyclic) resulting in skewing of the phenyl rings similar to the TCA’s Doxepine: dibenzoxepine an isostere of the TCA’s and all else is the same as the TC’s Amoxapine: Related to dibenzoxazepine antipsychotic loxapine without para methyl group which has been used in depressed psychotics with some success. Has more dopamine antagonistsic activity than some of the other antidepressants. Loxapine, interestingly, has little to no antidepressant activity Mirtazapine: a dibenzazepine but mechanistically is not related to the TCA’s at all. It is thought to be presynaptic a2 adrenergic receptor blocker that normally inhibit the release of the NE and 5-HT, thereby increasing active levels in the synapse. It also blocks post-synaptic 5HT2 and 5-HT3 receptors—thereby enhance serotonergic neurotransmission while causing a low incidence of side effects Metabilisme of mirtazapine Selective norepinephrine reuptake inhibitors (SNRI) Atomoxetine and Reboxetine are selective norepinephrine reuptake inhibitors (SNRIs) which are very selective for inhibiting the norepinephrine reuptake transporter (Be careful, SNRI has also been used to stand for Serotonin Norepinephrine reuptake inhibitor such as venlafaxine); These drugs lack a strong electron withdrawer on the phenyl ring that decreases selectivity for the serotonin transporter; They retain a similar side effect profile except adrenergic side effects (has more adrenergic side effects than SSRIs); Atomoxetine is used in the US for ADHD and Reboxetine is used in Europe for depression These drugs don’t have serotonergic side effects and decrease the risk for serotonin syndrome Metabolism of R-atomoxetine Selective serotonin reuptake inhibitors (SSRIs) SSRIs come from breaking the TCA ring structure that decreases bulk on the phenyl end The decreased bulk probably explains why these are not antagonists at the receptors that TCAs tend to antagonize (acetylcholine, norepinephrine, histamine) The group on the phenyl ring probably explains the selectivity for the serotonin transporter Fluoxetine (Prozac) was the first SSRI type antidepressant introduced (1986). Other examples include venlafaxine and duloxetine, which are SNRIs. Venlafaxine Duloxetine Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) Phenoxyphenylalkylamine selective serotonin reuptake inhibitors (SSRIs) Derivatives of fluoxetine Serotonine reuptake transporter (SERT) affinity Metabolism Phenylalkylamine SSRIs The metabolism of sertraline Miscellaneous SSRIs Non-tricyclic reuptake inhibitors Metabolism of venlafaxine Metabolism of duloxetine Dopamine and norepinephrine reuptake inhibitors; Bupropion Dopamine and norepinephrine reuptake inhibitors Serotonin reseptor modulators Metabolism of trazodone Metabolism of aripiprazole MAOI antidepresants MAOI antidepresants Isoniazide Antitubercular but too polar Iproniazide Antitubercular but CNS stimulant Which later shown to be MAOI resulting in NE & 5-HT New antidepressant introduced but withdrawn due to liver toxicity, however, increased interest on hydrazines and hydrazides for antidepressants H2N NH2 hydrazines Thus MAOIs based on the hydrazine molecule have been extensively studied. Hydrazine, itself, has no MAOI activity Must have a free amino at one end to be active; a protonatable terminal N is necessary; those without a terminal N are prodrugs and must be bioactivated Must have at least one free hydrogen on each nitrogen Adding an alkyl group to one nitrogen of hydrazine confers MAOI activity: Ethyl is the most potent of the series methyl, ethyl, propyl, etc. Branching with a methyl group does not affect potency MAOI antidepresants Ring Addition Adding a phenyl ring produces a compound with no MAOI activity Adding a benzyl ring confers good activity, adding a phenethyl (phenyl ethyl) ring is even more potent More closely approximates norepinephrine, serotonin and dopamine, etc Disubstitution Phenelzine N,N disubstitution on one end decreases, or loses, potency MAOI antidepresants Hydrazides: hydrazine with one nitrogen forming an amide, e.g., Iproniazid and isocarboxazide Isocarboxazide Iproniazid These drugs have lipophilic substituents that allow them to cross the BBB, where they are hydrolyzed to the active species. This increase the potency by allowing more drug to reach the site of action Note isoniazide is a hydrazide but is not bioactivated Mechanism of Action - MAOIs covalently bind MAO, irreversibly inhibiting the reaction. Phenelzine An effective mechanism-based antidepressant agent. It is presumably oxidized to diazene (HN=NH) (which can then break up into molecular nitrogen, a hydrogen atom), and a phenethyl free radical that would be the active species in irreversible inhibition Competitive MAOIs MAOI antidepresants The present clinically useful irreversible MAOIs are mechanism-based as they form reactants that covalently bond the enzyme or its cofactor Thus they may continue their action up to 2 weeks after administration is discontinued The harmala alkaloid harmaline and harmine are competitive inhibitors of MAO and are CNS stimulants Moclobemide has received considerable attention. It is an effective antidepressant without producing hypotensive crisis which is a reversible inhibitor of MAO-A and permits metabolism of dietary tyramine, however, caution is still needed to avoid excessive intake (of cheddar cheese, feva beans) MAOI antidepresants ► ► NH2 ► ► ► CH3 CH N C CH 3 CH3 N H CH3 Tranylcypromine The distance from the phenyl to the amine is closer to norepinephrine The cyclopropyl group is very strained (reactive) and alkylates the enzyme The trans isomer is more potent than the cis isomer The ring is more unstable and binds the enzyme better (-)-Selegiline Has an acetylene functional group that is unstable Results in covalent bond to the enzyme Part of its metabolic fate is N-dealkylation to methamphetamine Selegiline is the (–) isomer and so it forms (–) methamphetamine not the active (+) form as shown Selective MAO-B irreversible inhibitor (at lower doses) Lithium ion suppresses inositol triphosphate signaling Intracellular phosphatidylinositol signaling pathway and site of action for lithium Agomelatine The first approved antidepressant to incorporate a non-monoaminergic mechanism of action. Agomelatine The first approved antidepressant to incorporate a non-monoaminergic mechanism of action. Questions 1. Strukturerna för två läkemedel visas i Figur 2. Föreslå trolig farmakologisk effekt för varje läkemedel. 2. Läkemedel C kan metaboliseras till flera metaboliter. Rita och förklara hur tre olika metaboliter kan bildas. 3. D metaboliseras i huvudsak i levern, till den aktiva metaboliten X. Rita S-isomeren av metaboliten X. Questions Compound 2 and 3 are derivatives of compound 1. Will 2 and 3 have comparable pharmacological effect to 1? Questions Explain how the compound can be modified in order to obtain an improved SSRI