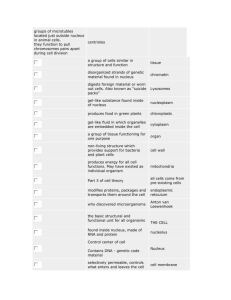
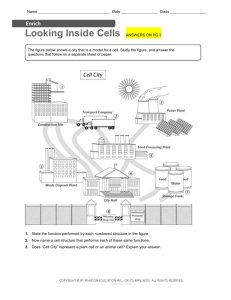
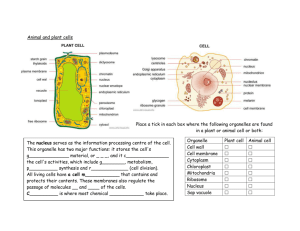
Acoustic stria: Anatomy of physiologically characterized
advertisement

THE JOURNAL OF COMPARATIVE NEUROLOGY 482:349 –371 (2005) Acoustic Stria: Anatomy of Physiologically Characterized Cells and Their Axonal Projection Patterns PHILIP H. SMITH,1* ANN MASSIE,2 AND PHILIP X. JORIS2 Department of Anatomy, University of Wisconsin, Medical School–Madison, Madison, Wisconsin 53706 2 Laboratory of Auditory Neurophysiology, K.U. Leuven, Medical School, B-3000 Leuven, Belgium 1 ABSTRACT The mammalian cochlear nucleus (CN) has been a model structure to study the relationship between physiological and morphological cell classes. Several issues remain, in particular with regard to the projection patterns and physiology of neurons that exit the CN dorsally via the dorsal (DAS), intermediate (IAS), and commissural stria. We studied these neurons physiologically and anatomically using the intra-axonal labeling method. Multipolar cells with onset chopper (OC) responses innervated the ipsilateral ventral and dorsal CN before exiting the CN via the commissural stria. Upon reaching the midline they turned caudally to innervate the opposite CN. No collaterals were seen innervating any olivary complex nuclei. Octopus cells typically showed onset responses with little or no sustained activity. The main axon used the IAS and followed one of two routes occasionally giving off olivary complex collaterals on their way to the contralateral ventral nucleus of the lateral lemniscus (VNLL). Here they can have elaborate terminal arbors that surround VNLL cells. Fusiform and giant cells have overlapping but not identical physiology. Fusiform but not giant cells typically show pauser or buildup responses. Axons of both cells exit via the DAS and take the same course to reach the contralateral IC without giving off any collaterals en route. J. Comp. Neurol. 482:349 –371, 2005. © 2005 Wiley-Liss, Inc. Indexing terms: onset choppers; octopus cells; giant cells; fusiform cells; cochlear nucleus; cat Projection neurons of the cochlear nucleus (CN) use two primary pathways to reach higher auditory structures (see Cant and Benson, 2003, for review). In the cat, the ventromedially situated trapezoid body (TB) carries axons arising primarily from bushy cells of the anteroventral cochlear nucleus (AVCN), which project to the superior olivary complex (SOC), and from multipolar cells throughout the ventral cochlear nucleus (VCN) that are classified as type 1 (Cant, 1981) and that project to the inferior colliculus. In other species cochlear root neurons also use this pathway (Lopez et al., 1999). A second pathway, the dorsally situated/directed acoustic stria, has three main components. The dorsal acoustic stria (DAS) is situated between the dorsal cochlear nucleus (DCN) and the caudal aspect of the posteroventral cochlear nucleus (PVCN) and carries the axons of the pyramidal (fusiform) and giant cells of the DCN from the CN to the contralateral inferior colliculus. Medial and slightly caudal to the DAS is the intermediate acoustic stria (IAS) primarily composed of the axons of VCN octopus cells that project to the con© 2005 WILEY-LISS, INC. tralateral ventral nucleus of the lateral lemniscus (VNLL). Finally, axons of type 2 multipolar cells that project across the brainstem to the opposite cochlear nucleus form a commissural stria whose axons are initially mingled with those of the IAS. As the IAS and commissural axons head dorsally out of the cochlear nucleus, the two populations separate, with the commissural axons running rostral to the IAS (Osen, 1969a,b). Grant sponsor: National Institutes of Health; Grant number: PO DC00116; Grant sponsor: National Science Foundation; Grant number: BNS-8901993; Grant sponsor: Fund for Scientific Research – Flanders; Grant number: G.0083.02 (to A.M., P.X.J., postdoctoral fellowship to A.M.); Grant sponsor: Research Fund K.U. Leuven (OT/01/42). *Correspondence to: Philip H. Smith, Dept. of Anatomy, University of Wisconsin, 1300 University Ave., Madison, WI 53706. E-mail: Smith@Physiology.wisc.edu Received 8 July 2004; Revised 27 August 2004; Accepted 8 October 2004 DOI 10.1002/cne.20407 Published online in Wiley InterScience (www.interscience.wiley.com). 350 P.H. SMITH ET AL. There have been several single-cell labeling studies in different species where individual members of the various subtypes of CN projection neurons were injected with either horseradish peroxidase (HRP) or Neurobiotin (Vector Labs, Burlingame, CA) (Rhode et al., 1983a,b; Rouiller and Ryugo, 1984; Smith and Rhode, 1985, 1987, 1989; Friauf and Ostwald, 1988; Ostapoff et al., 1994; Hancock and Voigt, 2002a,b; Palmer et al., 2003; Arnott et al., 2004). In these experiments the injection site has typically been at or close to the cell soma where the labeling substance may fill the cell body, dendritic tree, and local axon collaterals but rarely labels the axons past the boundaries of the CN. Studies in the trapezoid body (Spirou et al., 1990; Smith et al., 1991, 1993a,b) have successfully used intra-axonal recording methods to label the terminal portions of AVCN axons. Other than our preliminary report (Joris et al., 1992), no such study has been done on the acoustic stria. Thus, our knowledge about the projection patterns of axons using these pathways is based mainly on degeneration studies or retrograde and anterograde labeling studies where gross injections label populations of axons that are followed back to their cell bodies or forward to their termination sites (e.g. Warr, 1969, 1972, 1982; Adams and Warr, 1976; Adams, 1979; Schofield, 1995; Schofield and Cant, 1996a,b; for review, see Cant and Benson, 2003). In this study we used the intra-axonal recording and labeling method to get a more detailed account of the physiology, course, and termination sites of the cell populations that use the dorsal, intermediate, and commissural stria. MATERIALS AND METHODS The surgical approach and general stimulus and analysis procedures are as described in Joris (1998) and Joris and Smith (1998). The basic details for intra-axonal recording and labeling are as described previously for experiments on the trapezoid body and medial nucleus of the trapezoid body (Smith et al., 1991, 1993a, 1998). The experimental protocol was approved by the University of Wisconsin Animal Care and Use Committee and conforms to NIH guidelines. Young adult cats were anesthetized with intramuscular acepromazine (0.2 mg/kg) and ketamine (20 mg/kg) followed by intravenous infusion of sodium pentobarbital. A tracheal cannula was inserted. Rectal temperature was maintained at 37–38°C. Both pinnae were removed, the external auditory meati cut transversely, and metal earpieces inserted for delivery of acoustic stimuli. To maintain normal middle ear pressure polyethylene tubing was glued into a small hole made in each bulla. The posterior fossa was exposed and the cerebellum was carefully aspirated at the midline until the fourth ventricle was visible. Cerebellar aspiration was continued laterally until the dorsalmost aspect of left and right CN was noted. The striae could usually be visualized as they exited the CN and coursed over the restiform body as a thin, slightly raised band of tissue. To improve recording stability, a chamber was glued to the skull in order to attach a hydraulic microdrive for electrode advancement. A photograph of the area, including a millimeter scale for reference purposes, was taken to indicate penetration sites. Only one axon was injected in each penetration. The electrode was filled with buffered (pH 7.6) 2% Neurobiotin in 0.5 M KCl and was placed immediately above the stria as it curved over the restiform body. Warm 3% agar was poured over the region and allowed to solidify. Extracellular and intracellular recording used standard techniques for DC monitoring, amplification, filtering, and display and the records were continuously stored on magnetic tape. Entry into an axon was signaled by a DC shift from 30 to 50 mV (average 45 mV). After collection of physiological data, Neurobiotin was injected (500 ms pulses of 0.5–2 nA every second for 2–20 minutes, average duration 8 minutes), the electrode withdrawn from the brain, and the location and penetration depth recorded. The stria is very narrow in the rostrocaudal dimension as it leaves the cochlear nucleus and very shallow in the dorsoventral dimension. In addition, the stria briefly runs superficially over the top of the restiform body but then quickly dives below the brain surface. Nevertheless, it was usually possible to move a sufficient distance away from the first successful penetration to attempt one or two subsequent penetrations. If at least one injection was accomplished on one side, an injection was attempted on the opposite side of the brain. Acoustic stimuli and data collection Calibrated acoustic stimuli, under Digital Microvax computer control, were delivered through hollow earpieces connected to earphones (Radio Shack Super Tweeters). Spikes were converted to standard pulses with a peak detection circuit, which were sampled with a 1-s precision. The general stimulus paradigm was as follows. A search stimulus of tone bursts ranging from 100 – 40,000 Hz was presented until a unit was encountered. An automated tracking algorithm was used to obtain the threshold tuning curve from which characteristic frequency (CF, the frequency of lowest rate threshold), Q10 (a measure of sharpness of tuning), and spontaneous rate were determined. The responses of some cells, giant cells in particular (see Results), were too sluggish for the tracking algorithm to be practical. In such cases CF or BF (frequency of maximal response rate) was estimated with iso-SPL tone bursts stepped or swept in frequency. Responses to 25 or 50 ms duration tones at CF (STCF, interstimulus interval 100 or 200 ms, 200 repetitions, rise-fall times 3.9 ms) were obtained at various sound pressure levels (measured as decibels re. 20 Pa, dB SPL), usually in 10-dB steps, and visualized as rate-level functions and peristimulus time histograms (PSTHs). A rate-level function to digitally generated pseudorandom broadband noise bursts was also obtained (typical parameters: 100 ms bursts every 500 ms, 10-dB steps, 40 repetitions). On the basis of these data, particularly the shape of the PSTH at multiple suprathreshold levels, the cell was physiologically classified and it was decided whether we would attempt to label it. If time allowed, responses to additional stimuli, sometimes presented contralaterally, were presented to quantify temporal properties, including low-frequency tones, click trains at various rates, and amplitude-modulated tones (see Joris and Smith, 1998). Responses to these stimuli are not further discussed here. Tissue processing Perfusion, Neurobiotin reaction, and plastic embedding. After the last penetration the animal was maintained in an areflexive state for 18 –24 hours. Then, following a lethal dose of sodium pentobarbital, it was perfused with saline followed by two concentrations of ACOUSTIC STRIAL PROJECTION PATTERNS phosphate-buffered, calcium-containing glutaraldehyde/ paraformaldehyde fixative. The brain was then removed and stored overnight in the lower concentration fixative. Seventy-m sections were cut on a vibratome, the Neurobiotin visualized using the ABC reagent method (Vector Labs) and the Adams (1981) DAB-nickel/cobalt intensification method, and then prepared for light and/or electron microscopy (EM). For light microscopy, sections were mounted on glass slides, counterstained with cresyl violet, and coverslipped. For EM, the vibratomed, Neurobiotinreacted sections were fixed in 2% osmium tetroxide, dehydrated, and flat-embedded in Epon-Araldite plastic resin between two sheets of plastic film. After a camera lucida or a computer-aided 3D drawing of the injected axon and its parent cell body were made, section(s) containing pertinent portions of the injected cell were flat-mounted and resectioned into 5-m sections. The appropriate 5-m section was selected, remounted, trimmed, and thinsectioned, then counterstained with uranyl acetate and lead citrate and observed with a JEOL 100CX electron microscope. For viewing the axon in three dimensions we used the Neurolucida system (MicroBrightField, Colchester, VT), which allows the tracking of various structures through serial tissue sections. The outlines of the coronal brainstem sections containing the labeled axon were entered as well as labeled pieces of axon and various pertinent structures. These data were then merged to form a 3D image that could be rotated to any plane. Negatives of the electron micrographs were scanned on a Duoscan T1200 scanner (AGFA) using FotoLook software (AGFA) and any adjustments of tone or contrast were made using this software. Micrographs were then imported into Powerpoint (Microsoft) where figures were created. RESULTS This article focuses primarily on the anatomical rather than the physiological features of labeled cells whose axons use the acoustic stria. Only the basic physiological response properties of these cells will be described here. A more detailed account of the physiology of labeled and unlabeled axons will be presented in a subsequent article. Multipolar cells Onset choppers. The combined evidence from several different studies (Cant and Gaston, 1982; Wenthold, 1987; Kolston et al., 1992; Shore et al., 1992; Schofield and Cant, 1996b; Alibardi, 1998; Needham and Paolini, 2003; Palmer et al., 2003; Arnott et al., 2004) suggests that the large VCN multipolar cells whose response to short tones have been labeled onset-chopper (OC; Rhode et al., 1983a; Smith and Rhode, 1989) closely resemble a population of large glycinergic multipolar cells that project to the opposite CN. Single-cell labeling studies in the cat (Smith and Rhode, 1989) and guinea pig (Palmer et al., 2003; Arnott et al., 2004) have reported that the axons of these cells provide collateral innervation to both the ipsilateral dorsal and ventral cochlear nuclei before heading dorsally out of the CN. The only direct evidence that the glycinergic multipolar cells projecting to the opposite cochlear nucleus and the cells with OC response features are the same population is one juxtacellularly labeled cell in the guinea pig (Arnott et al., 2004) and one intra-axonally labeled cell in the cat that we described in a preliminary report (Joris 351 et al., 1992). That cell is included in the population described here. We labeled eight axons in the acoustic stria that showed clear onset-chopper response features consistent with the larger population described previously (Rhode et al., 1983a; Smith and Rhode, 1989). These axons could be traced retrogradely back to the cell body in the cochlear nucleus. The consistent physiological features of these cells to tones at CF included spike rates that increased over a wide dynamic range of intensities and the characteristic onset chopper PSTH. Figure 1 illustrates PSTHs of all eight of these labeled cells (Fig. 1C–G,I–K) as well as an OL response from another multipolar cell axon (Fig. 1H, see below) and an OC response from an unlabeled axon in the acoustic stria whose response was driven by contralateral stimulation (Fig. 1L). Tuning curves (Fig. 1A) and rate-level functions (Fig. 1B) of these same cells are also shown. All of the OC responses are characterized by regular firing at tone onset, generating a multipeaked histogram followed by no activity or a lower level of sustained, less regular activity. Figure 2 shows six examples (cells 1–5, 7) of the cell body and dendritic tree of OC cells and their locations within the cochlear nucleus and of one cell (Fig. 2, cell 6) with an OL PSTH. One of the OC cells was located within the deep DCN (Fig. 2, cell 1) while the rest were found at various rostrocaudal locations in the VCN. The location, cell size, and dendritic tree configuration of the OC cells in the VCN is in good agreement with the distribution of cells labeled in previous intracellular studies in the cat (Smith and Rhode, 1989) and with the location of most of the cells previously shown in gross injection studies to project to the opposite CN (Cant and Gaston, 1982; Shore et al., 1992; Schofield and Cant, 1996b; Alibardi, 1998). The gross injection studies also indicated that a few cells projecting to the opposite CN are in the deep DCN, which fits with our one labeled OC cell at this location. We examined three of these cells at the EM level to compare them with a previous report (Smith and Rhode, 1989). Both the cell body and proximal dendritic tree showed a dense synaptic coverage (Figs. 3A,B, 4A,D), a common feature of this cell type (Smith and Rhode, 1989). The axons of all the cells located in the VCN had collaterals in both the VCN and DCN. Most of the collateral field in the DCN innervated both fusiform and deep layers in and around the frequency region of the DCN that, based on reported frequency maps (Spirou et al., 1993), corresponds to the CF of the OC cell (Fig 3D). In the DCN the synaptic terminals could be seen on large dendrites as well as cell bodies (Fig. 3E) and contained nonround vesicles. The OC cell located in the deep DCN also had collateral branches that innervated the deep DCN and fusiform cell layer in and around the location of the parent cell body (Fig. 4C,E). We could not find any axon collaterals of this cell that headed for the VCN. After giving off collateral branches within the ipsilateral cochlear nucleus, the OC axon projected dorsally over the restiform body, with other strial axons. In all but two cases the axon could also be traced in an anterograde direction from the injection site just dorsal to the restiform body. Axons that could be followed ran medially and rostrally within the DAS bundle of fibers crossing cranial nerve VII just below the genu (Fig. 5B,C) and then rostrally, medially, and ventrally toward the midline. After crossing the midline dorsal to the superior olivary com- 352 Fig. 1. Tuning curves (A), rate-level functions (B), and PST histograms to short tones at CF of nine labeled multipolar cells (C–K) and one contralaterally driven unlabeled axon with OC physiology (L). The OC responses (C–G,I–L) show regular firing at tone onset that generates multiple peaks followed by no activity or a lower level of sustained, less regular spiking. The OL PSTH (H) shows a single onset peak followed by a pause and a resumption of spontaneous activity. CFs (in kHz) are indicated on the PST histograms, which were obtained at 74 or 84 dB SPL, except for the high-threshold cell (E), where the level was 104 dB. Linestyles and symbols at CFs in A correspond with the other panels. For clarity of illustration, not all datapoints in B are plotted with a symbol. PST histograms have 500 bins. The morphology of the cells from which the first seven PST histograms were derived is shown in the same order in Figure 2. P.H. SMITH ET AL. plex, the axons of the DAS continued rostrally, just lateral to the contralateral lateral lemniscus, whereas five of the OC axons instead turned and headed caudally and laterally toward the opposite cochlear nucleus, a trajectory best appreciated in the horizontal plane (Fig. 5E). In two cases we were able to follow the axon as it headed under the spinal trigeminal tract and nucleus and into the contralateral cochlear nucleus (Fig. 5D), where it began branching before fading. In three cases the axon faded just before entering the cochlear nucleus. None of these “typical” OC axons gave off any collaterals to nuclei in the superior olivary complex. The axons of two multipolar cells in the VCN with OC physiology did not conform to this typical contralateral pathway. One axon (Fig. 2, cell 7) had “typical” collaterals in the ipsilateral cochlear nucleus and followed the same course as the OC axons described above until it reached the midline. Just after crossing the midline dorsal to the superior olivary complex it headed straight ventrally, within the confines of a single 70-m section, to assume a position in the ventral aspect of the trapezoid body, and then crossed back over the midline to run caudally under the MSO and LSO in the trapezoid body, where it faded, heading toward the parent cochlear nucleus. A second cell (Fig. 2, cell 5) with OC physiology sent an axon across the ipsilateral brainstem after giving off collaterals in the ipsilateral CN and following the same course as the “typical” OC axons. After crossing the midline the axon branched as it approached the dorsomedial end of the contralateral MSO. The main axon (based on diameter) headed ventrally and around the medial side of the MSO and then coursed dorsally and rostrally until it became too light to follow lateral to the VNLL. The collateral branch headed over the contralateral MSO and faded medial to the rostral end of the MSO. OL multipolar cell. We labeled one cell whose physiology differed somewhat from the OC multipolar cell population described above. The PSTH of this cell (Fig. 1H) shows a single onset peak followed by a pause and then a low level of sustained activity; this pattern is often referred to as onset with low sustained activity (OL). In addition, the rate intensity function was compressive. The large cell body was located in the VCN and the dendritic tree was multipolar, like the OCs (Fig. 2, cell 6). At the EM level the cell body resembled a type 2 multipolar cell in that a large percentage of the surface of the cell body and proximal dendrites was covered with synaptic terminals (64%, Fig. 6A,B). The axon was unusual in that it showed characteristics of both OC and octopus cell axons. It headed dorsally into the acoustic stria and gave off collaterals to the DCN (like an OC axon). At the EM level the terminals contained nonround vesicles and synapsed on dendrites and cell bodies in the DCN much like the OCs terminals described above (Fig. 6C,D). The axon continued dorsally out of the cochlear nucleus via the DAS and, after crossing the midline, it started heading caudally (like an OC axon). However, just after getting to the contralateral side it gave off a collateral that went to the contralateral DMPO (like some octopus cells). The main axon continued caudally and laterally (like an OC) over the contralateral LSO, then, just lateral to the contralateral LSO, gave off a small but distinct collateral that headed into the contralateral cochlear nucleus (like an OC but usually the main axon heads there). The main axon turned rostrally to Fig. 2. Camera lucida representations of seven large stellate cells in the CN, six with OC PSTHs (cells 1–5, 7) and one with a OL PSTH (cell 6), and their location in sections of the cochlear nucleus. Asterisks in sections indicate the cell body location: four in the AVCN/nerve root area, two in the PVCN, and one in the deep DCN. Arrows indicate axons. Cells and dendritic trees are oriented in the figure as they are in the sections. AN, auditory nerve; AVCN, anteroventral cochlear nucleus; DCN, dorsal cochlear nucleus; OCA, octopus cell area; PVCN, posteroventral cochlear nucleus. Scale bar in 1 ⫽ 1 mm (applies to all drawings of tissue sections); scale bar in 5 ⫽ 100 m (applies to all cell drawings). Fig. 3. Electron microscopic features of an onset chopper unit. A: Micrograph of the labeled cell body (CB, cell 4 in Fig. 2). B: Electron micrograph of a primary dendrite (d) illustrating the dense synaptic coverage of the dendritic tree. C: Camera lucida drawings of the cell body and dendritic tree. D: Axon collaterals innervating the DCN. Arrows indicate path of an orthodromic spike. The main terminal field is confined to a fairly narrow region of the deep and fusiform cell layer of the DCN. The two insets illustrate the location of the terminal field (gray areas) in two representative sections of the DCN. Arrows indicate small pieces of the main axon in each section. E: Micrograph of labeled synaptic terminals (asterisks) from this DCN terminal field synapsing on a dendrite (d) and a small sparsely innervated cell body (CB) in the deep DCN. Scale bars ⫽ 5 m in A; 2 m in B; 1 mm in C; 200 m in D; 2 m in E. ACOUSTIC STRIAL PROJECTION PATTERNS reach the contralateral VNLL where it started branching/ innervating this area (like an octopus cell axon). Octopus cells Octopus cells (Osen, 1969) have large tentacle-like dendrites which often arise from one side of the cell body and are oriented in one direction. They are located exclusively in the posteriormost aspect of the PVCN in an area designated the octopus cell area (OCA), and recordings here (Godfrey et al., 1975) indicate that these cells typically respond to short tones with an onset response. Response features from only three positively identified octopus cells have ever been reported in the cat. In one, the axon faded just after leaving the CN (Rhode et al., 1983a). In a second the cell was so lightly labeled that an axon could not be identified (Rouiller and Ryugo, 1984). The other was described in our preliminary report (Joris et al., 1992) and is included here. Thus, the information on octopus cell axonal projections comes primarily from retrograde and anterograde gross injection studies (e.g., Warr, 1969; Adams, 1997). We labeled the three octopus cell axons that could be traced back to their cell body and two axons that could not be traced back to the cell body but whose physiology and axonal projection pattern would lead us to believe that they belong to octopus cells. Figure 7A illustrates tuning curves and PST histograms of the three octopus cells and the two axons. All cells showed onset responses followed by a low level or no sustained response. For four of these the onset response was very well timed and the sustained activity very low; for these the PSTH can be classified as OI (onset with a very low level of sustained activity). For the fifth cell (top right PSTH) the CF exceeded the equipment calibrated frequency range and we were only able to use tone stimulation that was at most 15 dB above threshold; possibly, the PSTH would have been more like the other cells if higher stimulus levels could have been used. Several other important features of these cells, including their remarkable abilities to respond to short repetitive stimuli at very high rates, will be included in a subsequent article. Of the three recovered cell bodies, one was very darkly labeled and virtually all of the dendritic tree could be distinguished (Fig. 7B, cell 1). A second cell body was moderately well labeled and much of the primary and secondary dendritic tree could be distinguished (Fig. 7B, cell 2). The third cell was lightly labeled and only the primary and initial portions of the secondary dendrites could be seen (Fig. 7C, cell 3). All were in the OCA and had the typical oriented dendritic trees. In order to compare the synaptic inputs to these cells with descriptions in a previous report (Kane, 1973), EM of the most darkly labeled cell body and its primary dendrites was done. The cell body and primary dendrites showed significant synaptic terminal coverage (60%), with many resembling auditory nerve terminals (Fig. 8A–C). Before leaving the CN, the axon of the darkly labeled cell (Fig. 7B, cell 1, arrow) gave off two small collaterals which innervated the interstitial nucleus of the stria of Held (Warr, 1969). The axons of the other two cells were lightly labeled while in the CN, so although we saw no local collaterals, we cannot rule out the possibility that they were too light to distinguish. All three axons headed dorsally in the acoustic stria. After coursing over the restiform body the axons separated from the DAS/ commissural pathway by turning sharply ventral while 355 slowly heading rostrally medial to the restiform body. From this point the axons followed one of two paths on their way to a common target, the VNLL. The course of one of these axons is shown in the coronal and horizontal plane in Figure 8D. Two of them (one is illustrated in Fig. 8D) ran medially while still heading rostrally dorsal to ipsilateral LSO and MSO. One of them continued ventrally and rostrally and ran beneath the LSO before heading dorsally again between the LSO and MSO. All three then continued medially and rostrally over and past the ipsilateral MSO. In two cases we could see ipsilateral collaterals. One axon had a collateral above the LSO which innervated the dorsolateral periolivary group. Just medial to this the same axon gave off a second collateral that headed ventrally, directly between the LSO and MSO before going under the MSO and branching into the region just medial to it. A second octopus cell axon gave off a collateral quite dorsal in the ipsilateral brainstem which could not be followed to its termination. The two axons whose cell bodies were not labeled but were most likely octopus cell axons were too lightly labeled on the ipsilateral side to see collaterals. The main axons of the three octopus cells and the two tentative octopus cell axons continued medially and rostrally crossing the midline dorsal to the superior olivary complex. On the contralateral side the axons headed rostrally and laterally going over MSO and LSO. Two of these axons gave off collaterals that headed into the DMPO and one gave off a collateral that headed toward the DLPO before fading. All five axons continued rostrally into the contralateral lateral lemniscus and could be followed into the VNLL before fading; three could be seen branching in the VNLL. The collaterals of one very well-labeled axon in the VNLL are illustrated in Figure 9. The large myelinated axon (Fig. 9A) began branching as it approached the VNLL (Fig. 9B). Some of the axon terminals were large. EM of one of these revealed that the terminal provided multiple synaptic contacts containing round synaptic vesicles (Fig. 9C) on a cell body in the VNLL (Fig. 9D) in a fashion similar to globular bushy calyceal terminals on cells in the MNTB. DCN fusiform and giant cells When recordings are made in the acoustic stria, the predominant PSTH pattern encountered is the pauser or buildup pattern (P/B, an onset peak followed by a pause of variable length then a resumption of spike activity (P), or no onset and a buildup of spike activity after some delay (B)). The association, in the pentobarbital-anesthetized cat, of these PSTH patterns with fusiform cells was first proposed based on extracellular recordings (Kiang et al., 1965; Godfrey et al., 1975) and is now well established through intracellular labeling (Rhode et al., 1983b; Smith and Rhode, 1985; Ding et al., 1999; Hancock and Voigt, 2002a,b). Less well established is the physiology of giant cells. It has been concluded from extracellular recordings that DCN giant cells have the same physiology as fusiform cells because 1) in deep DCN stable responses can be obtained which are indistinguishable from responses obtained in the fusiform cell layer (Godfrey et al., 1975; Young and Brownell, 1976; Voigt and Young, 1988; Spirou and Young, 1991), and 2) DCN projection cells that can be antidromically activated from the DAS have the same physiological properties as cells in the fusiform cell layer (Young, 1980). Oddly, some of our very first successfully labeled axons were from giant cells whose responses were Figure 4. ACOUSTIC STRIAL PROJECTION PATTERNS 357 Fig. 5. Course of the axon of one of the onset chopper units (cell 4 in Fig. 2) out of the cochlear nucleus, across the brainstem, and into the contralateral cochlear nucleus. A: Section containing the cell body. Asterisk and arrow indicate the location of the cell body. B–D: Coronal sections illustrating the course of the axon as it heads across the brain stem. Asterisks and arrows point to the small pieces of the axon found in each of the sections. E: Frontal (lower) and horizontal (upper) sections illustrating the course of the whole axon (arrows) across the brainstem. F: Camera lucida drawing of the cell body and dendritic tree. Cochlear nucleus containing the cell body is labeled “ipsi.” AS, acoustic stria; CB, cerebellum; D, dorsal; G, genu of cranial nerve seven; IV, fourth ventricle; IVN, inferior vestibular nucleus; LSO, lateral superior olive; LVN, lateral vestibular nucleus; M, medial; MNTB, medial nucleus of the trapezoid body; MSO, medial superior olive; PT, pyramidal tract; R, rostral; RB, restiform body; STN, spinal trigeminal nucleus; STT, spinal trigeminal tract; VI, cranial nerve six; VII, cranial nerve seven. Other abbreviations as in Fig. 2. Scale bar ⫽ 1 mm in D (applies to A–D); 2 mm in E; 100 m in F. Fig. 4. Electron microscopic features of an onset chopper unit located in the DCN. A: Micrograph of the labeled cell body (CB) in the deep DCN (cell 1 in Fig. 2). B: Camera lucida drawing of the cell body and dendritic tree of this cell. C: Camera lucida drawing of the cell body with most of the dendritic tree removed showing the axon collateral field of the cell in the DCN. Arrows indicate direction of orthodromic spike. D: Micrograph of a primary dendrite of the cell (d) illustrating the typical dense synaptic coverage. E: Micrograph of one of the labeled terminals (asterisk) from this DCN terminal field synapsing on a dendrite in the fusiform cell layer. Scale bars ⫽ 5 m in A; 100 m in B; 250 m in C; 2 m in D,E. very different from those of fusiform cells. To increase our yield of labeled giant cells we therefore attempted injections primarily of axons with “unusual” responses. Labeling of axons with clear P/B responses was only attempted as a last resort (i.e., when several penetrations over several hours had not resulted in impalement of non-P/B fibers). Our sample of labeled neurons is thus biased physiologically and possibly also anatomically. We labeled 15 axons that could be traced back to fusiform cells in the DCN and eight axons that were traced Fig. 6. Electron microscopic features of the OL unit. A: Electron micrograph (left) and camera lucida (right) of a primary dendrite (d) illustrating the dense synaptic coverage. B: Electron micrograph (left) and camera lucida (right) of the cell body (CB) illustrating the dense synaptic innervation. C,D: Electron micrographs of two examples of the cell’s synaptic terminals (asterisks) synapsing on a dendrite (d) and a cell body (CB) in the deep DCN. Scale bars ⫽ 2 m in A; 10 m in B; 2 m C (applies to C,D). ACOUSTIC STRIAL PROJECTION PATTERNS 359 Fig. 7. A: Octopus cells and their response features. Top left: Threshold tuning curves for the three labeled octopus cells and two labeled octopus cell axons. The tuning curves were smoothed with a 3-point filter to reduce overlap, but symbols are placed at the CF and threshold measured on the unsmoothed tuning curve. For cell 1 (triangle) the CF is taken to be the highest frequency within the calibration range to which the cell responded. PSTHs 1–3 are obtained from the corresponding cells in B. PSTHs 4 and 5 are from the labeled axons. Stimulus SPL was: (1) 89, (2) 79, (3) 79, (4) 80, (5) 70 dB. PSTHs have 500 bins. The numbers on the PSTHs indicate CF (in kHz) and the symbol to the appropriate tuning curve. B: Camera lucida representations of three octopus cells in the CN and their location in sections of the cochlear nucleus. Asterisks in sections indicate cell body location. Arrows indicate axons. Cells and dendritic trees are oriented in the figure as they are in the sections. Scale bar ⫽ 100 m in 2 (applies to all cell drawings); 1 mm in 1 (applies to all drawings of the tissue sections). back to giant cells in the deep DCN. Figure 10 illustrates the physiology of eight labeled fusiform cells to short tone bursts. The tuning curves (Fig. 10A) show that these cells have low thresholds to tones (note that for two cells the CF was above the calibration limit of our equipment, so that the absolute threshold is unknown). A second important property is that the rate-level curves to short CF tones typically show a nonmonotonicity at intermediate SPLs (Fig. 10B). The PSTHs shown are at SPLs in this range. Five labeled fusiform cells showed the P/B pattern that is typically observed in the pentobarbital-anesthetized cat. PSTHs for four of these cells are shown in Figure 10C–F. In seven of the labeled cells the pause following the onset peak was shorter and less profound (Fig. 10G–I). Nevertheless, their PSTHs still show some resemblance to the P/B pattern. Figure 10J illustrates a fusiform cell response that was unusual in that it had a well-timed chopping pattern at all SPLs. Another unusual cell (not illustrated) had a high threshold (⬃70 dB), gave poor responses to tones, and was inhibited by broadband noise. Figure 11 illustrates the physiology of seven labeled giant cells. This physiology showed considerable diversity that cannot be captured in a unified description. Moreover, very limited data are available from these cells because the contact time with these fibers tended to be short and also because of the physiological response properties themselves. In very broad terms, the online evaluation of these cells indicated high CFs, poor frequency tuning, high thresholds, a predominance of inhibition rather than excitation, and sluggish responses with low and regular Fig. 8. Electron microscopy of an octopus cell and axonal course. A: Electron micrograph of the cell body (CB, cell 1 in Fig. 7). B: Electron micrograph of a proximal dendrite (d). C: Drawings of the cell body and dendrite from the sections in A and B illustrating the dense synaptic coverage of these regions, a common feature of this cell type. D: Frontal (upper) and horizontal (lower) sections illustrating the course of the whole axon (arrows) across the brainstem. Cochlear nucleus containing the cell body is labeled “ipsi.” Abbreviations as in Figure 5. Scale bars ⫽ 10 m in A,B; 2 mm in D. Fig. 9. Octopus cell innervation of the contralateral VNLL. A: Electron micrograph of the myelinated axon (asterisk). B: Camera lucida drawing of the axon collateral system within the VNLL. Curved arrow indicates location of terminal shown in D. C: Electron micrograph showing an octopus cell synaptic terminal on a cell body (CB) and spine (s) in the VNLL. D: Low-power electron micrograph of a cell in the VNLL (CB) receiving multiple synaptic terminals (arrows) from the octopus cell axon. Scale bars ⫽ 100 m in B; 1 m in C (applies to A,C); 10 m in D. 362 Fig. 10. Physiology of eight labeled fusiform cells. A: Threshold tuning curves of the cells whose peristimulus time histograms are shown in C–J. B: Rate intensity functions of the cells whose RAs and PSTHs are shown in A and C–J. C–J: PSTHs to 200 short tones at CF. CFs (in kHz) are indicated on the PST histograms, which were obtained at SPLs between 54 and 69 dB. Line styles and symbols at CFs in A correspond with the other panels. For clarity of illustration, the tuning curve tails in A were removed, and not all datapoints in B are plotted with a symbol. For two cells (H,I) CF was above the upper frequency limit for which calibrated tonal stimuli could be delivered. firing rates. Figure 11A illustrates the high thresholds and wide tuning. In four cells a tuning curve could not be obtained due to the sluggishness of the response, and iso-level tones were used to obtain an estimate of frequency tuning (Fig. 11B,E,I). With one exception (Fig. 11H), none of these cells showed the characteristic P/B pattern and nonmonotonic behavior typical of fusiform cells in this preparation. By the same token, the PSTHs P.H. SMITH ET AL. Fig. 11. Response features recorded from seven labeled giant cell axons in the acoustic stria. A: Tuning curves for four giant cells. The symbols indicate CF and are keyed to the corresponding PST histograms in panels D (89 dB), F (74 dB), G (74 dB), and H (84 dB). B,C: The iso-intensity (74 dB) response rate at different frequencies and the PST histogram at the BF (35 kHz, 84 dB) of one neuron. There was no response at 64 dB. E: a set of iso-intensity contours at 34, 54, and 74 dB for a giant cell that showed only inhibitory responses. There was no response at 34 dB. I,J: response rate to a frequency sweep and a PST histogram at BF (42 kHz, 64 dB) for one cell. The sweep rate was 20 kHz/sec and extended from 10 Hz to 40 kHz and back at 90 dB SPL. The dashed lines in B, E, I indicate spontaneous rate. Numbers above PST histograms indicate the stimulus frequency. The ordinate of all panels, except A, is firing rate in spikes/ sec. certainly fall in the range of “atypical” response patterns that can be observed in fusiform cells (Fig. 10G–J). Several of the giant cells show sequential modes in their PSTHs, i.e., “chopping.” In two cases with high spontaneous activity (Fig. 11B,C,J) the response to broadband noise was inhibitory at threshold but excitatory at higher levels. ACOUSTIC STRIAL PROJECTION PATTERNS Thus, with the necessarily limited sample size of labeled cells available, it appears that there are global differences in physiological properties of giant vs. fusiform cells, but that there is also overlap, so that for a given cell the physiology is less predictive of anatomical type than for several other CN cell classes. Cells with low thresholds, clear P/B pattern and a nonmonotonic rate curve are likely to have fusiform morphology. Cells with high thresholds and sluggish responses are more likely to have giant morphology. The fusiform cells were located in the fusiform cell layer. Cell body locations relative to their best frequencies agreed with a previous description of the frequency map of the cat fusiform cell layer (Spirou et al., 1993). Light microscopic anatomy of these cells and their dendritic trees conformed to the descriptions of labeled cat fusiform cells from previous reports (Rhode et al., 1983b; Smith and Rhode, 1985) and are not illustrated here. Giant cells were located deep to the fusiform cell layer. Figure 12 illustrates five giant cells and their locations in the deep DCN. Cells 2 and 4 in Figure 12 were darkly labeled and the majority of the dendritic tree appeared to be labeled. Cells 3 and 5 were moderately well-labeled so distal dendrites were most likely too light to see. Cell 1 was lightly labeled so that only proximal dendrites could be visualized. We did EM on the cell bodies of three giant cells and one fusiform cell to compare the terminal coverage with previous reports (Smith and Rhode, 1985; Kane et al., 1981). Two of the giant cell bodies (Fig. 13A,B) and the fusiform cell body (Fig. 13D) showed synaptic coverage that was not as dense as the octopus and OC cell bodies (43%, 44%, 39%); however, one of the giant cells (Fig. 13C) had significant coverage (62%). The axons of both fusiform and giant cells joined the acoustic stria in the cochlear nucleus and headed dorsally. We occasionally saw collaterals coming off of fusiform cell axons within the deep DCN, as reported previously (Rhode et al., 1983b; Smith and Rhode, 1985), but never saw giant cell collaterals within the ipsilateral DCN. Many of the axons were not darkly labeled while in the CN, so we could have missed such collaterals. We followed 15 fusiform axons and six giant cell axons across the brainstem. The axons of both cell types followed a similar course heading over the restiform body and coursing medially and rostrally toward the midline within the DAS. Figures 14 and 15 illustrate examples of the course of giant and fusiform cell axons. In Figure 14, representative sections show the location of small pieces of one giant cell axon as it heads across the brainstem and into the IC. Figure 15 shows the entire course of a giant cell axon (Fig. 15A) and a fusiform cell axon (Fig. 15B) from two separate experiments in frontal, horizontal, and sagittal planes. Also shown in Figure 15C is the course of a giant and fusiform axon whose cell bodies were in the same cochlear nucleus. On the contralateral side the axons continued rostrally and laterally dorsal to the LSO and MSO then joined the lateral lemniscus just medial to the VNLL and continued dorsally just medial to the dorsal nucleus of the lateral lemniscus (DNLL). The axons of these two cell types never gave off any collaterals within the brainstem to any of the auditory nuclei of the superior olivary complex or the lemniscal nuclei. Four of the fusiform cell axons and two of the giant cell axons faded medial to the DNLL just below the IC, while six fusiform and two giant axons entered the ventral aspect of the IC. Unfortunately, these axons could 363 not be followed past the initial branch points within the IC. DISCUSSION The data presented here clarify some of the anatomical details of physiologically characterized members of the major classes of projection neurons of the cochlear nucleus whose axons exit via the acoustic stria. Onset choppers Two features indicate that the major role of the onset chopper on the ipsilateral side of the brainstem is to provide a wide-band inhibitory influence on other cell types in the cochlear nucleus. First, the axon collateral system of the OC cells has terminals with features of inhibitory synapses that innervate both the VCN and DCN. Second, studies have indicated that several cell types within the ipsilateral CN are inhibited by a short latency input, with features consistent with the OC output. One such cell type is the multipolar cell classified by different authors as type 1, T, or planar (Cant, 1981; Oertel et al., 1990; Doucet and Ryugo, 1997; Alibardi, 1999; Doucet et al., 1999). These type 1 cells: 1) have axons that give off collaterals to the DCN before projecting to the IC via the trapezoid body; 2) have axon terminals containing small round vesicles; 3) have sparsely innervated cell bodies; 4) show chopping PSTHs in response to tones; and 5) are narrowly tuned in their frequency response. Smith and Rhode (1989) provided anatomical evidence that the OCs synapse on the type 1 multipolar cells in the cat PVCN. In the rodent, Ferragamo et al. (1998) showed that the D stellates, believed to be the rodent equivalent of OCs, provide glycinergic inhibition to the type 1 multipolars. Pressnitzer et al. (2001) reported that transient choppers in the AVCN, believed to be type 1 multipolars, receive a wide-band inhibition that may arise from OCs. OC projections to the DCN have also been implicated in the wide-band glycinergic inhibition demonstrated physiologically in DCN principal cells, as well as in the cells designated type II or vertical (Caspary et al., 1987; Young et al., 1992; Nelken and Young, 1994; Backoff et al., 1997; Joris and Smith, 1998; Spirou et al., 1999; Davis and Young, 2000; Anderson and Young, 2004). Our electron microscopy of OC terminals in the deep DCN shows that they can synapse on cell bodies or dendrites in this region, which is consistent with a potential influence of the OCs on these cell types. In all but two cases we could follow the axons of these cells dorsally out of the CN and across the brainstem. In one of the two cases the axon heavily innervated the DCN before heading into the stria. At the injection site a large axon branch was also seen heading back down the stria into the tuberculoventral tract to innervate the VCN. In the second case the axon headed into the stria and just before the injection site gave off a collateral that headed back into the cochlear nucleus to innervate the DCN. The observed branching and “looping back” could explain the observation (Kolston et al., 1992) that the commissural stria, at the level where it exits the DCN, contains both small and large diameter glycine-positive axons. The smaller axons might be collaterals looping back. The inability, in these two cases, to follow the main axon out of the cochlear nucleus has at least two possible explanations: 1) these axons only doubled back into the home CN 364 Fig. 12. Camera lucida representations of five giant cells in the deep DCN and their locations in sections of the cochlear nucleus. Arrows indicate axons. Asterisks indicate cell body locations in the DCN. Cells and dendritic trees are oriented in the figure as they are in the sections. One of the cells (#1) was lightly labeled so the den- P.H. SMITH ET AL. dritic tree could not be followed past the primary dendrites. Much of the dendritic tree of the other cells could be seen. Physiology of the five cells is shown in the following panels of Figure 11: 1 (B,C), 2 (A,D), 3 (E), 4 (A,F), 5 (A,G). Scale bars ⫽ 1 mm in 3 (applies to all drawings of tissue sections); 100 m in 4 (applies to all cell drawings). ACOUSTIC STRIAL PROJECTION PATTERNS Fig. 13. Electron microscopy of the cell bodies of giant and fusiform cells. A–C: Left: Electron micrographs of labeled giant cell bodies. The cells in A and B are cells 3 and 5 in Figure 12. A camera lucida drawing of the cell in C is not illustrated in Figure 11. D: Electron microscopy of a fusiform cell body. Right: Drawings of the cell bodies and synaptic terminals on the cells from the sections illustrated on the left. Scale bar ⫽ 10 m (applies to all micrographs). and did not leave it, or 2) the projection part of the axon did not fill past the injection site. The second option is brought up because it has been our experience that, on rare occasions, when we labeled other cell populations intra-axonally, the axon would only label in one direction 365 from the injection site. This is a rare event but may be explained by the fact that the axon can pull apart at the injection site and form “constriction bulbs” at the two free ends of the break. For example, we have labeled globular bushy cells, whose axons are known to project out of the cochlear nucleus, for which only one labeled constricted end of the axon could be found and followed back to the cell body, while nothing was found in the anterograde direction. Thus, it is possible that some axons pull apart during the recording and simply do not fill past the injection site. Again, this is a rare observation and it should be noted that Arnott et al. (2004) reported two large multipolar cells that were labeled juxtacellularly whose axons did not appear to leave the cochlear nucleus (see below). This method labels the cell at the cell body, so constriction of the axon would not be a plausible explanation for their observation. While heading across the brainstem to the opposite CN, the majority of our labeled OC axons followed a consistent course. On this course these typical OC axons did not target any of the superior olivary or paraolivary nuclei either ipsilaterally or contralaterally, which is in agreement with the single labeled OC axon reported by Arnott et al. (2004) in the guinea pig. We also noted that there is some diversity in the path taken by these axons across the brainstem, and perhaps the structures innervated by them. Two of our axons did not follow this standard course upon reaching the contralateral side. In one case the axon turned back at the midline and appeared to be returning to the home CN, but faded before its target could be distinguished. A second axon followed the standard course across the ipsilateral brainstem, but then branched within the superior olivary complex contralaterally. Neither branch appeared to turn and head caudally. Unfortunately, both these branches faded, so they could have been headed for any structure, including the opposite cochlear nucleus by a different route. Our labeled axons entered the opposite cochlear nucleus by heading under the spinal trigeminal nucleus and tract and entering the ventral division of the cochlear nucleus. After making gross injections, Cant and Gaston (1982) noted that many of the labeled commissural axons use this pathway. They also noted that some of the axons appear to enter the opposite cochlear nucleus dorsally via the acoustic stria. In support of this finding, we recorded one axon in the stria (see Fig. 1, bottom right, PSTH) that was driven by the contralateral ear and had an OC response pattern with short latency. The primary projection of the OC axon contralaterally is to the CN, where, like the ipsilateral projection, it appears to be providing a wide-band inhibition over the entire CN to several of the major cell groups (Cant and Gaston, 1982; Schofield and Cant, 1996; Babalian et al., 1999, 2002; Alibardi, 2000; Shore et al., 2003). These may include both type 1 and 2 multipolar, globular and spherical bushy, octopus, and fusiform cells. As we (Joris and Smith, 1998) and others have discussed previously, the contralateral inhibition at the level of the cochlear nucleus has certain features that are consistent with known properties of OC neurons, namely, a stronger inhibition to noise than to tones and a short latency. Despite these findings of binaural effects at the level of the CN, the functional role of this contralateral inhibition remains obscure. For example, despite the ability of the OC cell category to display excellent envelope phase-locking, the inhibitory response 366 P.H. SMITH ET AL. Fig. 14. Course of one of the giant cells axons out of the cochlear nucleus, across the brainstem, and into the contralateral inferior colliculus. A: The left section shows the CB location in the cochlear nucleus (asterisk), the middle picture is the cell itself (cell 3 in Fig. 11). The axon headed caudally before heading out of the cochlear nucleus. The right section in A shows the axon at its most caudal location (asterisk and arrow). B–E: Sections illustrating the course of the axon as it heads across the brain stem. Asterisks and arrows point to the small pieces of the axon found in each of the sections. BC, brachium conjunctivum; BP, brachium pontis; IC, inferior colliculus; ICO, commissure of the inferior colliculi; PGL, lateral pontine gray; PGM, medial pontine gray; TB, trapezoid body; TRC, tegmental reticular nucleus; 7N, nucleus of cranial nerve seven. Other abbreviations as in Figure 5. Scale bar ⫽ 1 mm (applies to drawings of all sections). of their postsynaptic targets to this well-timed input is sluggish and temporally unremarkable (Joris and Smith, 1998). Second, where contralateral auditory stimulation has been presented in the presence of ipsilateral auditory stimulation, the reduction in firing due to the contralateral inhibition is easily overcome by ipsilateral excitation (Young and Brownell, 1976; Joris and Smith, 1998; Recio, pers. commun.). On-Ls A classification scheme was recently developed (Winter and Palmer, 1995) for distinguishing two forms of onset cells in the guinea pig: OCs and OLs. For both sets of cells the steady state response at 20 dB above threshold is less than 50 sp/sec and the onset to steady-state ratio greater than 10. To subdivide these onset cell types, features of the onset response were considered. Units were classified as OL if, in response to tones, there was a single onset peak followed by a pause in firing and then a resumption of firing at a low level. Units were classified as OC if there were multiple onset peaks and no pause before any sustained activity. Recent work in this same lab (Arnott et al., 2004) has shown that juxtacellularly labeled multipolar cells with OL response features had axons that did not appear to project out of the CN. Based on the physiological criteria described above, one of our labeled stellates could be classified as OL (Fig. 2, cell 6). This cell had some anatomical features resembling the OC cell population, ACOUSTIC STRIAL PROJECTION PATTERNS 367 Fig. 15. Giant and fusiform cell axon course. A: Frontal (top), horizontal (middle), and sagittal (bottom) views of the course of a giant cell axon across the brainstem to the contralateral inferior colliculus. B: Frontal (top), horizontal (middle), and sagittal (top) views of the course of a fusiform cell axon across the brainstem to the contralateral inferior colliculus. C: Frontal (top) and horizontal (bottom) views of the course of a fusiform and giant axon across the same brainstem. Cochlear nucleus containing the cell body is labeled “ipsi.” NLL, nuclei of the lateral lemniscus. Other abbreviations as in Figure 5. Scale bar ⫽ 2 mm in B (applies to all drawings). but others that did not. Unfortunately, since we have only a single example of this cell type, the question remains as to whether there is a distinct set of OC and OL multipolar cells that perform different functions. Octopus cells Although our sample is small, we provide the first examples of physiologically characterized octopus cells whose cell body and axonal projection pattern were recovered and analyzed. Four responded to tones at their CF with a well-timed onset response that could be classified as OI. One had a less well-timed onset response but the CF of this cell was too high to deliver stimuli at levels well above threshold, which generally improves the timing of the first spike. The cell bodies were all located in the OCA. Electron microscopy of one of the labeled cell bodies and proximal dendrites showed dense synaptic coverage, a feature that has been previously described (Kane, 1973). The axons headed out of the cochlear nucleus and across the brainstem to innervate the contralateral VNLL and 368 P.H. SMITH ET AL. the terminals here could form multiple synapses on a single cell body. As a population, the octopus axons innervate several periolivary nuclei, but individual members may innervate none, one, or more of these nuclei. In vitro experiments on octopus cells by Oertel and colleagues (Golding et al., 1995, 1999; Gardner et al., 2001; Oertel et al., 2000) have shown that octopus cells have very low input resistances, short time constants, and rapid glutamate receptor kinetics that make them well suited for detecting coincident arrivals of synaptic inputs from many small auditory nerve terminals and responding only when they arrive in precise register. This is supported by the responses of these cells to click trains and amplitude-modulated stimuli (Godfrey et al., 1975; Rhode, 1994), which we have also observed and will report in a subsequent article. It is interesting to note the anatomical and physiological similarities between these octopus cells and globular bushy cells in the AVCN. Slice data have shown that both cell types have nonlinear membrane features that allows them to generate only a single spike in response to constant depolarizing current (Oertel, 1983; Golding et al., 1995), and to follow short, synchronous synaptic inputs at high rates. Anatomically, the axons of both cell types are also similar (see Cant and Benson, 2003, for review). Neither axon provides substantial innervation within the cochlear nucleus (Smith and Rhode, 1987, this study). Axons of both cell types are large and project out of the cochlear nucleus to consistently influence the principal cells of one brainstem nucleus: the VNLL by octopus cells, and the MNTB by bushy cells. The endings of these axons in the MNTB and VNLL are large and excitatory and generate multiple terminations directly onto cell bodies. Extracellular recordings from these postsynaptic cells show prepotentials that represent the activity within the large axon terminal. The terminal recipient cells in the MNTB and VNLL are inhibitory and have been shown to have nonlinear membrane properties similar to their CN input, firing only once to depolarizing current pulses (Banks and Smith, 1992; Wu, 1999; Zhao and Wu, 2001). Fusiform and giant cells While one morphological class of DCN projection neurons—the fusiform (or pyramidal) cells— has been particularly well studied, the other morphological class is probably the least studied projection neuron of the entire CN. In contrast to other CN principal cells, giant cells have not been associated with a distinct response pattern. Extensive extracellular studies in the decerebrate cat by Young and colleagues (reviewed by Young and Davis, 2002) have not revealed any dichotomous differences that may be associated with the two types of DCN principal neurons, and these authors have therefore concluded that the two types of neurons do not differ in their physiology. This is remarkable because these cells are so different in their morphology and location in the layered DCN, and thus presumably also in their inputs. We provide the first in vivo data from morphologically identified giant cells. The data are limited and fragmented because the recording times were generally short and because it was difficult to find stimuli to drive the cells. Giant cells tended to show high thresholds, high spontaneous activity that could be inhibited by tones over a wide frequency range, sluggish and irregular responses, and a bias to very high frequencies. The responses of only one case (Fig. 11H) showed the key features displayed by the majority of fusiform cells, i.e., P/B PSTHs, a strongly nonmonotonic rate curve to STCF, and an excitatory response to broadband noise. On the other hand, some of the labeled cells which lacked the key features just mentioned turned out to be fusiform cells (Fig. 10G–J), although they did have the expected better tonal thresholds. Thus, our data do not provide physiological signatures allowing unequivocal distinction between the two cell types, but they nevertheless strongly suggest that the two populations differ in their physiology: the archetypal P/B pattern and its associated properties predominates in fusiform cells, but not in giant cells. EM of the cell body of one fusiform cell showed fairly sparse synaptic coverage, in agreement with previous data on these cells (Smith and Rhode, 1985). EM of three of our labeled giant cells indicates that they may receive a sparse somatic innervation but can receive innervation that is as dense as that seen on octopus or OC cell bodies. Kane et al. (1981) also reported a variable innervation density between members of the giant cell population. Our sample is too small and our data too fragmented to determine whether giant cell terminal density is correlated with any anatomical or physiological parameter of this population. The axons of both cell types leave the cochlear nucleus via the DAS. Some fusiform axons show collaterals within the DCN, but we saw no giant cell axon collaterals. Unfortunately, these axons were often lightly labeled so we cannot say with certainty that they did not have collaterals and did not influence other cells in the CN. The main fusiform or giant cell axon takes a similar course on their way into the lateral lemniscus. Along this course many of the axons were darkly labeled, yet we never saw any collaterals given off to the brainstem auditory nuclei in the olivary complex or the nuclei of lateral lemniscus, so it is clear that the DCN output has no direct effect on neurons interposed between the CN and the inferior colliculus. Both sets of axons entered the IC but faded before the branching pattern could be distinguished, so we were unable to carefully study individual axonal innervation patterns or determine whether any of the axons continued to the thalamus. Malmierca et al. (2002) have shown in the rat that at least some of the giant cells and perhaps some of the fusiform cells in the DCN project to the medial division of the auditory thalamus. Whether this is a subpopulation of the giant and fusiform cells and whether these same cells also synapse in the IC is unknown. What might be the function of these projection neurons of the DCN? Several studies approached this question by lesioning output of the DCN to look at its effect on behavior or the response of downstream targets (Bengry et al., 1977; Casseday and Neff, 1975; Jenkins and Masterton, 1982; Masterton and Granger, 1988). Sutherland et al. (1998a,b) and May (2000) reported that such a lesion disrupted sound orientation behavior. Young and colleagues (Young et al., 1995; Davis et al., 1996; Kanold and Young, 2001) have shown that the DCN projection neurons respond not only to auditory stimuli but to somatosensory inputs from muscle proprioceptors in and around the pinna as well. Such evidence has led to speculation that the DCN output may be involved in coordinating pinna orientation and localization cues found in the different spectra of sounds located at different points in space (see review, Young and Davis, 2002). ACOUSTIC STRIAL PROJECTION PATTERNS At present, it is not known whether the fusiform and giant cells perform the same or different functions. Oliver et al. (1997) showed that innervation in the IC from the DCN overlaps with contralateral LSO innervation. Although both sets of DCN principal cells are presumed to be generating this innervation pattern, there is no evidence that fusiform and giant axons influence the same or different cells in the region. Ramachandran et al. (1999) showed that some IC cells, designated type O, had response features similar to fusiform and giant cells and speculated that some of these cells were getting these response features from their DCN inputs. Davis and colleagues (Davis, 2002; Davis et al., 2003) provided evidence that a subclass of the type O category, the low rate type Os, receive direct input from cells using the DAS and speculated that this pathway may be part of a circuit that is specialized to detect spectral features. Again, none of these studies were able to distinguish whether giant cell axons, fusiform axons, or both, contributed to the observations. One piece of anatomical data may provide an initial indication that the two DCN output cell types have different functions. This is based on a unique synaptic input to the fusiform cells that shows plasticity. Fusiform cells have both a spiny apical dendritic tree extending into the superficial DCN layers and a set of nonspiny basal dendrites extending into the deep DCN. Like the fusiform basal dendrites, giant cell dendrites are aspinous and confined primarily to the deep DCN, where the primary inputs are from auditory nerve fibers. In the superficial DCN layers, fusiform apical dendrites receive parallel fiber inputs from granule cells that have been shown to display activity-dependent plasticity, while the auditory nerve input does not (Fujino and Oertel, 2003). Thus, even if the fusiform and giant cell axons projected to exactly the same sites/cells, one input (the fusiforms) might have a response that could vary, depending on the conditions, while the other (giants) might lack this ability. ACKNOWLEDGMENTS We thank J.P. Timmermans and collaborators at the University of Antwerp for enabling the 3D axonal tracing. We dedicate this article to Kirsten Osen, an enormous figure in cochlear nucleus anatomy who many years ago first suggested to us the possible link between the onset chopper category and the commissural cells. LITERATURE CITED Adams JC. 1979. Ascending projections to the inferior colliculus. J Comp Neurol 183:519 –538. Adams JC. 1981. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 29:775. Adams JC. 1997. Projections from octopus cells of the posteroventral cochlear nucleus to the ventral nucleus of the lateral lemniscus in cat and human. Aud Neurosci 3:335–350. Adams JC, Warr WB. 1976. Origin of axons in the cat’s acoustic striae determined by injection of horseradish peroxidase into severed tracts. J Comp Neurol 170:107–122. Alibardi L. 1998. Ultrastructural and immunocytochemical characterization of commissural neurons in the ventral cochlear nucleus of the rat. Ann Anat 180:427– 438. Alibardi L. 1999. Fine structure, synaptology and immunocytochemistry of large neurons in the rat dorsal cochlear nucleus connected to the inferior colliculus. J Hirnforsch 39:429 – 439 Alibardi L. 2000. Putative commissural and collicular axo-somatic termi- 369 nals on neurons of the rat ventral cochlear nucleus. J Submicrosc Cytol Pathol 32:555–566. Anderson MJ, Young ED. 2004. Isoflurane/N2O anesthesia suppresses narrowband but not wideband inhibition in dorsal cochlear nucleus. Hear Res 188:29 – 41. Arnott RH, Wallace MN, Shackleton TM, Palmer AR. 2004. Onset neurons in the anteroventral cochlear nucleus project to the dorsal cochlear nucleus. J Assoc Res Otolaryngol Online publication. Babalian AL, Ryugo DK, Vischer MW, Rouiller EM. 1999. Inhibitory synaptic interactions between cochlear nuclei: evidence from an in vitro whole brain study. Neuroreport 10:1913–1917. Babalian AL, Jacomme AV, Doucet JR, Ryugo DK, Rouiller EM. 2002. Commissural glycinergic inhibition of bushy and stellate cells in the anteroventral cochlear nucleus. Neuroreport 13:555–558. Backoff PM, Palombi PS, Caspary DM. 1997. Glycinergic and GABAergic inputs affect short-term suppression in the cochlear nucleus. Hear Res 110:155–163. Banks MI, Smith PH. 1992. Intracellular recordings from neurobiotinlabeled cells in brain slices of the rat medial nucleus of the trapezoid body. J Neurosci 12:2819 –2837. Bengry MF, Silverman MS, Clopton BM. 1977. Effects of lesioning the dorsal and intermediate acoustic striae on binaural interaction at the inferior colliculus. Exp Brain Res 28:211–219. Cant NB. 1981. The fine structure of two types of stellate cells in the anterior division of anteroventral cochlear nucleus. Neuroscience 6:2643–2655. Cant NB, Benson CG. 2003. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nucleus. Brain Res Bull 60:457– 474. Cant NB, Gaston KC. 1982. Pathways connecting the right and left cochlear nuclei. J Comp Neurol 212:313–326. Caspary DM, Pazara KE, Kossl M, Faingold CL. 1987. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res 417:273–282. Casseday JH, Neff WD. 1975. Auditory localization: role of auditory pathways in brainstem of cat. J Neurophysiol 38:842– 858. Davis KA. 2002. Evidence of a functionally segregated pathway from dorsal cochlear nucleus to inferior colliculus. J Neurophysiol 87:1824 –1835. Davis KA, Young ED. 2000. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol 83:926 –940. Davis KA, Miller R, Young ED. 1996. Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus. J Neurophysiol 76:3012–3024. Davis KA, Ramachandran R, May BJ. 2003. Auditory processing of spectral cues for sound localization in the inferior colliculus. J Assoc Res Otolaryngol 4:148 –163. Ding J, Benson TE, Voigt HF. 1999. Acoustic and current-pulse responses of identified neurons in the dorsal cochlear nucleus of unanesthetized, decerebrate gerbils. J Neurophysiol 82:3434 –3457. Doucet JR, Ryugo DK. 1997. Projections from the ventral cochlear nucleus to the dorsal cochlear nucleus in rats. J Comp Neurol 385:245–264. Doucet JR, Ross AT, Gillespie MB, Ryugo DK. 1999. Glycine immunoreactivity of multipolar neurons in the ventral cochlear nucleus which project to the dorsal cochlear nucleus. J Comp Neurol 408:515–531. Ferragamo MJ, Golding NL, Oertel D. 1998. Synaptic inputs to stellate cells in the ventral cochlear nucleus. J Neurophysiol 79:51– 63. Friauf E, Ostwald J. 1988. Divergent projections of physiologically characterized rat ventral cochlear nucleus neurons as shown by intraaxonal injection of horseradish peroxidase. Exp Brain Res 73:263–284. Fujino K, Oertel D. 2003. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci U S A 100:265–270. Gardner SM, Trussell LO, Oertel D. 2001. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci 21:7428 –7437. Godfrey DA, Kiang NYS, Norris BE. 1975. Single unit activity in the posteroventral cochlear nucleus of the cat. J Comp Neurol 162:247– 268. Golding NL, Robertson D, Oertel D. 1995. Recordings from slices indicate that octopus cells of the cochlear nucleus detect coincident firing of auditory nerve fibers with temporal precision. J Neurosci 15:3138 – 3154. Golding NL, Ferragamo MJ, Oertel D. 1999. Role of intrinsic conductances 370 underlying responses to transients in octopus cells of the cochlear nucleus. J Neurosci 19:2897–2905. Hancock KE, Voigt HF. 2002a. Intracellularly labeled fusiform cells in dorsal cochlear nucleus of the gerbil. I. Physiological response properties. J Neurophysiol 87:2505–2519. Hancock KE, Voigt HF. 2002b. Intracellularly labeled fusiform cells in dorsal cochlear nucleus of the gerbil. II. Comparison of physiology and anatomy. J Neurophysiol 87:2520 –2530. Jenkins WM, Masterton RB. 1982. Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol 47:987–1016. Joris PX. 1998. Response classes in the dorsal cochlear nucleus and its output tract in the chloralose-anesthetized cat. J Neurosci 18:3955– 3966. Joris PX, Smith PH. 1998. Temporal and binaural properties in dorsal cochlear nucleus and its output tract. J Neurosci 18:10157–10170. Joris PX, Smith PH, Yin TCT. 1992. Responses and projections of dorsal and intermediate stria axons labeled with HRP or Neurobiotin. Assoc Res Otolaryngol Abstr 14:56. Kane EC. 1973. Octopus cells in the cochlear nucleus of the cat: Heterotypic synapses upon homeotypic neurons. J Neurosci 5:251–279. Kanold PO, Young ED. 2001. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci 21:7848 –7858. Kiang NY, Pfeiffer RR, Warr WB, Backus AS. 1965. Stimulus coding in the cochlear nucleus. Trans Am Otolog Soc 53:35–58. Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J. 1992. An atlas of glycine- and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat Embryol 186:443– 465. Lopez DE, Saldana E, Nodal FR, Merchan MA, Warr WB. 1999. Projections of cochlear root neurons, sentinels of the rat auditory pathway. J Comp Neurol 415:160 –174. Malmierca MS, Merchan MA, Henkel CK, Oliver DL. 2002. Direct projections from cochlear nuclear complex to auditory thalamus in the rat. J Neurosci 22:10891–10897. Masterton, RB, Granger EM. 1988. Role of the acoustic stria in hearing: contribution of dorsal and intermediate striae to detection of noises and tones. J Neurophysiol 60:1841–1860. May BJ. 2000. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res 148:74 – 87. Needham K, Paolini AG. 2003. Fast inhibition underlies the transmission of auditory information between cochlear nuclei. J Neurosci 23:6357– 6361. Nelken I, Young ED. 1994. Two separate inhibitory mechanisms shape the responses of dorsal cochlear nucleus type IV units to narrowband and wideband stimuli. J Neurophysiol 71:2446 –2462. Oertel D. 1983. Synaptic responses and electrical properties of cells in brain slices of the mouse anteroventral cochlear nucleus. J Neurosci 3:2043–2053. Oertel D, Wu SH, Garb MW, Dizack C. 1990. Morphology and physiology of cells in slice preparations of the posteroventral cochlear nucleus of mice. J Comp Neurol 295:136 –154. Oertel D, Bal R, Gardner SM, Smith PH, Joris PX. 2000. Detection of synchrony in the activity of auditory nerve fibers by octopus cells of the mammalian cochlear nucleus. Proc Natl Acad Sci U S A 97:11773– 11779. Oliver DL, Beckius GE, Bishop DC, Kuwada S. 1997. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol 382:215–229. Osen KK. 1969a. Cytoachitecture of the cochlear nuclei in the cat. J Comp Neurol 136:453– 484. Osen KK. 1969b. The intrinsic organization of the cochlear nuclei of the cat. Acta Oto-laryngol 67:352–359. Ostapoff EM, Feng JJ, Morest DK. 1994. A physiological and structural study of neuron types in the cochlear nucleus. II. Neuron types and their structural correlation with response properties. J Comp Neurol 346:19 – 42. Palmer AR, Wallace MN, Arnott RH, Shackleton TM. 2003. Morphology of physiologically characterized ventral cochlear nucleus stellate cells. Exp Brain Res 153:418 – 426. Pressnitzer D, Meddis R, Delahaye R, Winter IM. 2001. Physiological correlates of comodulation masking release in the mammalian ventral cochlear nucleus. J Neurosci 21:6377– 6386. P.H. SMITH ET AL. Ramachandran R, Davis KA, May BJ. 1999. Single-unit responses in the inferior colliculus of decerebrate cats. I. Classification based on frequency response maps. J Neurophysiol 82:152–163. Rhode WS. 1994. Temporal coding of 200% amplitude modulated signals in the ventral cochlear nucleus of the cat. Hear Res 77:43– 68. Rhode WS, Smith PH. 1986. Anatomical and physiological studies of a class of wide dynamic range neurons in the cat cochlear nucleus. Neuroscience Abstr 12:1266. Rhode WS, Oertel D, Smith PH. 1983a. Physiological response properties of cells stained intracellularly with horseradish peroxidase in the cat ventral cochlear nucleus. J Comp Neurol 213:448 – 463. Rhode WS, Smith PH, Oertel D. 1983b. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol 213:426 – 447. Rouiller EM, Ryugo DK. 1984. Intracellular marking of physiologically characterized cells in the ventral cochlear nucleus of the cat. J Comp Neurol 225:167–186. Schofield BR. 1995. Projections from the cochlear nucleus to the superior paraolivary nucleus in guinea pigs. J Comp Neurol 360:135–149. Schofield BR, Cant NB. 1996a. Projections from the ventral cochlear nucleus to the inferior colliculus and the contralateral cochlear nucleus in guinea pigs. Hear Res 102:1–14. Schofield, BR, Cant NB. 1996b. Origins and targets of commissural connections between the cochlear nuclei in guinea pigs. J Comp Neurol 375:128 –146. Shore SE, Godfrey DA, Helfert RH, Altschuler RA, Bledsoe SC Jr. 1992. Connections between the cochlear nuclei in guinea pig. Hear Res 62: 16 –26. Shore SE, Sumner CJ, Bledsoe SC, Lu J. 2003. Effects of contralateral sound stimulation on unit activity of ventral cochlear nucleus neurons. Exp Brain Res 153:427– 435. Smith PH, Rhode WS. 1985. Electron microscopic features of physiologically characterized, HRP-labeled fusiform cells in the cat dorsal cochlear nucleus. J Comp Neurol 237:127–143. Smith PH, Rhode WS. 1987. Characterization of HRP-labeled globular bushy cells in the cat anteroventral cochlear nucleus. J Comp Neurol 266:360 –375. Smith PH, Rhode WS. 1989. Structural and functional properties distinguish two types of multipolar cells in the ventral cochlear nucleus. J Comp Neurol 282:595– 616. Smith, PH, Yin TCT, Joris PX, Carney L. 1991. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol 304:387– 407. Smith PH, Joris PX, Banks MI, Yin TCT. 1993a. Responses of cochlear nucleus neurons and projections of their axons. In: Merchan M, Juis JM, Godfrey DA, editors. The mammalian cochlear nuclei: organization and function. New York: Plenum. p 349 –360. Smith PH, Joris PX, Yin TC. 1993b. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J Comp Neurol 331:245–260. Smith PH, Joris PX, Yin TC. 1998. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol 79:3127–3142. Spirou GA, Young ED. 1991. Organization of dorsal cochlear nucleus type IV unit response maps and their relationship to activation by bandlimited noise. J Neurophysiol 66:1750 –1768. Spirou GA, Brownell WE, Zidanic M. 1990. Recordings from cat trapezoid body and HRP labeling of globular bushy cell axons. J Neurophysiol 63:1169 –1190. Spirou GA, May BJ, Wright DD, Ryugo DK. 1993. Frequency organization of the dorsal cochlear nucleus in cats. J Comp Neurol 329:36 –52. Spirou GA, Davis KA, Nelken I, Young ED. 1999. Spectral integration by type II interneurons in dorsal cochlear nucleus. J Neurophysiol 82: 648 – 663. Sutherland DP, Glendenning KK, Masterton RB. 1998a. Role of acoustic striae in hearing: discrimination of sound-source elevation. Hear Res 120:86 –108. Sutherland DP, Masterton RB, Glendenning KK. 1998b. Role of acoustic striae in hearing: reflexive responses to elevated sound-sources. Behav Brain Res 97:1–12. Voigt HF, Young ED. 1988. Neural correlations in the dorsal cochlear nucleus: pairs of units with similar response properties. J Neurophysiol 59:1014 –1032. ACOUSTIC STRIAL PROJECTION PATTERNS Warr WB. 1969. Fiber degeneration following lesions in the posteroventral cochlear nucleus of the cat. Exp Neurol 23:140 –155. Warr WB. 1972. Fiber degeneration following lesions in the multipolar and globular cell areas in the ventral cochlear nucleus of the cat. Brain Res 40:247–270. Warr WB. 1982. Parallel ascending pathways from the cochlear nucleus: neuroanatomical evidence of functional specialization. Contrib Sens Physiol 7:1–38. Wenthold RJ. 1987. Evidence for a glycinergic pathway connecting the two cochlear nuclei: an immunocytochemical and retrograde transport study. Brain Res 415:183–187. Winter IM, Palmer AR. 1995. Level dependence of cochlear nucleus onset unit responses and facilitation by second tones or broadband noise. J Neurophysiol 73:141–159. Wu SH. 1999. Physiological properties of neurons in the ventral nucleus of the lateral lemniscus of the rat: intrinsic membrane properties and synaptic responses. J Neurophysiol 81:2862–2874. 371 Young ED. 1980. Identification of response properties of ascending axons from dorsal cochlear nucleus. Brain Res 200:23–37. Young ED, Brownell WE. 1976. Responses to tones and noise of single cells in dorsal cochlear nucleus of unanesthetized cats. J Neurophysiol 39: 282–300. Young ED, Davis KA. 2002. Circuitry and function of the dorsal cochlear nucleus. In: Oertel D, Popper AN, Fay RR, editors. Integrative functions in the mammalian auditory pathway. New York: Springer. p 160 –206. Young ED, Spirou GA, Rice JJ, Voigt HF. 1992. Neural organization and responses to complex stimuli in the dorsal cochlear nucleus. Philos Trans R Soc Lond B 336:407– 413. Young ED, Nelken I, Conley RA. 1995. Somatosensory effects on neurons in dorsal cochlear nucleus. J Neurophysiol 73:743–765. Zhao M, Wu SH. 2001. Morphology and physiology of neurons in the ventral nucleus of the lateral lemniscus in rat brain slices. J Comp Neurol 433:255–271.