CYP450-2C9 - New York State Council of Health
advertisement

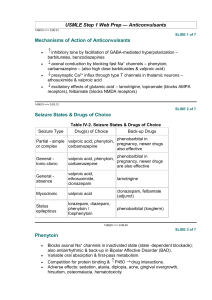
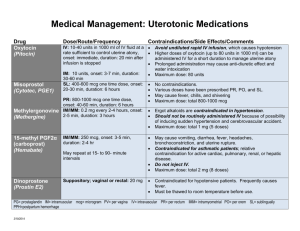
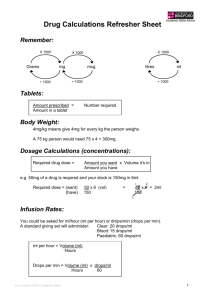
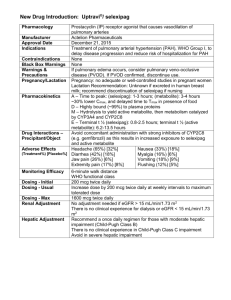
A Primer on Applied Antiepileptic Clinical Pharmacokinetics Henry Cohen, BS, MS, PharmD, FCCM, BCPP, CGP Professor of Pharmacy Practice Arnold & Marie Schwartz College of Pharmacy and Health Sciences of Long Island University and Chief Pharmacotherapy Officer Director of Pharmacy Residency Programs (PGY-1 & PGY-2) Department of Pharmacy Kingsbrook Jewish Medical Center Brooklyn, New York Phenytoin Pharmacokinetics Weak acid MW = 252 Daltons  F = 1 (all dosage forms)  T1/2 = concentration dependent    7 – 40 hours Vd = 0.65 l/kg ABW  90% protein bound  > 95% Metabolized via CYP450-2C9 to inactive metabolite 5-p-hydroxyphenyl-5phenylhydantoin 2C19 metabolism 5-10%   < 5% unchanged in urine Enterohepatic recycling CYP450-2C9 Effect of CYP2C9 Genotype on Phenytoin  CYP-2C9*1 is the active wild type allele  Primarily metabolized by CYP 2C9, and to a  CYP-2C9*2 or CYP-2C9*3 are inactive variant alleles lesser extent 2C19  Small changes in metabolism yield significant changes in serum levels 35% of Caucasians have variant alleles Michaelis Menten Pharmacokinetics *1/*1 genotype = 314 mg/day *2/*2 genotype = 217 mg/day *3/*3 genotype = 150 mg/day – Poor metabolizers (PMs) 1 – 3% African Americans and Asians Absent in 1% of Caucasians and African-Americans – *3/*3 genotype (homozygotes) Phenytoin Levels Michaelis-Menten Kinetics  Target serum level: 10 – 20 mcg/mL  80% response rate 90% response with levels 15 mcg/mL  Some patients respond to 5 – 10 mcg/mL  Target free serum level: 1 – 2 mcg/mL  Free concentration in CSF, bile, saliva, semen, breast milk, and GI fluids is the same as in blood Mixed-Order Kinetics A drug that goes from 1st Order elimination to Zero Order elimination  1st order at low concentrations  Zero Order at high concentrations  Phenytoin Michaelis-Menten Pharmacokinetics in a 70 kg Patient Phenytoin 100 mg 200 mg 300 mg 400 mg 500 mg 600 mg 700 mg 1st Order [50%] 5 mg/L 10 mg/L 15 mg/L    35 mg/L 60 mg/L 90 mg/L 120 mg/L Empiric Phenytoin Dosing Based on a Steady State Level 125 mg/5 mL Orange color Orange/Vanilla Flavor Must Shake Well Immediately before use 1 - 2 minutes Use water rinsings Avoid bottle – error prone Consider unit dose cups Consider syringes Phenytoin IV (Dilantin®)  Used in addition to iv benzodiazepines for Status Traditionally used concomitantly with diazepam Limited efficacy for Status Epilepticus – too slow!  Digoxin-Induced Ventricular Dysrhythmias  More effective than lidocaine Reverses Digoxin-induced AV node conduction depression  Dissolved in a solvent containing 40% Propylene Glycol 10% Ethanol Adjusted to pH 12 with NaOH ® Phenytek 0 Order [5 mg/kg] Phenytoin Suspension  Dilantin® Measured Phenytoin Serum Concentration Suggested Dosage Increase < 7 mg/L 100 mg/day or more 7 – 12 mg/L 50 mg/day >12 mg/L 30 mg/day Phenytoin IV (Dilantin®) Dosing  Empiric Dose: 18 – 20 mg/Kg ABW Onset within 15 – 60 minutes Use LD equation and target 20 mcg/mL Dilute 250 mg in 50 – 100 mL NS or ½ NS – 1 g in 250 mL NS – May dilute 1:1 if necessary Not compatible with D5W Maximum rate of administration is 50 mg/minute Stable for 4 hours Must use 0.22 micron in-line filter to remove crystals and minimize phlebitis Phenytoin IV  Phenytoin Oral Loading Cardiotoxic – due to propylene glycol?? Hypotension Bradycardia PR & QRS Prolongation Ventricular fibrillation Continuous cardiac monitoring  Onset: 6 – 10 hours to achieve target  Use suspension (F = 1; S = 1)  400 mg as highest dose every 2 hours  Doses of 400 mg or less minimize GI toxicity – Cardiac monitor must be in place – Is a cardiac monitor required for MD (100 mg – 200 mg IVPB)?? No?  Doses above 400 mg increase tmax Do not exceed 10 – 20 mg/minute Phenytoin Oral Loading  Conditions Altering Phenytoin Protein Binding Hypoalbuminemia LD of 600 mg 300 mg stat; and 300 mg in 2 hours 400 mg stat; and 200 mg in 2 hours  LD of 1g  LD of 1.2 g Hepatic Disease Hyperbilirubinemia Warfarin Nephrotic Syndrome Jaundice Valproic Acid Elderly Liver disease >2 g ASA Malnourished (AIDS) Diarrhea Renal dysfunction NSAIDs 400 mg stat; 300mg q2 hours for 2 doses 400 mg stat and q2 hours for 3 total doses  LD of 1.5 g Displacement by Displacement by endogenous cmpds Exogenous Cmpds Burns 300 mg stat and q 2 hours for 5 total doses Trauma Cystic Fibrosis Pregnancy The Sheiner-Tozer Equation For patients with Qcr ≥ 25 mL/min Ccorrected = Phenytoin Cobserved 0.2 (Albumin) + 0.1 For patients with Qcr ≤ 15 mL/min Phenytoin Cobserved Ccorrected = (0.1) (Albumin) + 0.1 1. Amino acid sequence is altered 2. Uremic solute displaces phenytoin 3. Protein wasting nephropathy 4. Free Phenytoin is 0.2 – 0.35 5. Phenytoin is 65 – 80% protein bound The Accuracy of the Sheiner-Tozer Equation Ref n Patients Prediction Results Temp Beck 49 28 – 81 yo No bias in formula 25°C Dager 29 28 - 79yo Underpredicted PHT by 12.4% Half < 2 mg/L 37°C Mylnarek 17 Critically ill 9% error Mauro 23 Critically ill Underpredicted by 16 ± 19% 25°C Cohen 15 Geriatric Underpredicted by 10.7 ± 5% 37°C Half < 1 mg/L 37°C?? Case . 70 yo, 70 kg, 6’, WM, on Phenytoin ER Capsules 100 mg qid; Creatinine Clearance = 35 mL/minute Albumin = 1 g/dL Phenytoin level at steady state is 13 mg/L Patient is seizure free – should a dose adjustment be made? Ccorrected = Phenytoin Cobserved Case. 60 yo, 60 kg, 5’5’, BF, on Phenytoin ER Capsules 100 mg qid Creatinine Clearance = 5 mL/minute Albumin = 1 g/dL Phenytoin level at steady state is 17 mg/L Patient is seizure free – should a dose adjustment be made? Ccorrected = 0.25 (Albumin) + 0.1 0.1 (Albumin) + 0.1 13 mg/L Ccorrected = 17 mg/L Ccorrected = (0.25) (1 g/dl) + 0.1 Ccorrected = 37 mg/L (0.1) (1 g/dl) + 0.1 Cobserved = 85 mg/L Fosphenytoin Conversion to Phenytoin Fosphenytoin (Cerebyx®)  Phenytoin Cobserved Prodrug of phenytoin Possesses a disodium phosphate ester that is cleaved during hydrolysis Converted by ubiquitous phosphatases T1/2 of conversion = 15 minutes  1 mg phenytoin sodium equivalent units is therapeutically equivalent to 1.5 mg of fosphenytoin Na 1.5 mg Fosphenytoin = 1 mg PE 1 g phenytoin = 1.5 g fosphenytoin Always dose using phenytoin equivalents or PE  Water soluble; pH 8.6 – 9 Mean plasma-free phenytoin concentration (mcg/mL) IV CEREBYX® (fosphenytoin) Absorption and Bioavailability CEREBYX® (fosphenytoin) vs Phenytoin Infusion Rates and Times Free phenytoin concentrations attained with infusion of 1200 mg PE CEREBYX at 150 mg PE/min versus 1200 mg IV phenytoin at 50 mg/min Infusion Time 3.5 1200 mg PE IV CEREBYX at 150 mg PE/min 3 (n=12) 1200 mg IV phenytoin at 50 mg/min 2.5 Phenytoin Equivalent Dose 2 1.5 CEREBYX (fosphenytoin) 150 mg PE/min IV Phenytoin 50 mg/min* 750 mg 5 min 15 min 1000 mg 6.6 min 20 min 1250 mg 8.3 min 25 min 1500 mg 10 min 30 min 1 Therapeutic level of free phenytoin 0.5 0 0 10 20 30 40 50 60 Time after start of infusion (min) *IV Phenytoin Prescribing Information. In a 3-way crossover study, 12 healthy subjects received 1200 mg IV phenytoin infused at 50 mg/min and 1200 mg PE fosphenytoin at rates from 50 to 150 mg PE/min Please see full Prescribing Information for CEREBYX®. Fosphenytoin (Cerebyx®) Carbamazepine Indication & Levels  Indicated for Partial, GTC, Mixed Seizures Highly bound to plasma proteins  Displaces phenytoin Transient high phenytoin levels Trigeminal Neuralgia and Bipolar Disorders  Target Levels are 4 – 12 mg/L May infuse at 150 mg/minute (max)  >8 mg/L CNS ADRs increase 11 – 15 mg/L: drowsiness, nystagmus and ataxia 15 – 25 mg/L: Combativeness, hallucinations, chorea >25 mg/L: Coma and seizures Phlebitis, pain and burning (No IV filter) Pruritus, paresthesia – perineal area  Time to reach target level (>1 mcg/mL)  May administer IM IV: 5 minutes; IM: 24 minutes Oxcarbazepine (Trileptal®): No Epoxide, No Autoinduction O OH N N O O NH2 N NH2 O O Oxidation N NH2 NH2 MHD (Active) Oxcarbazepine O No autoinduction Gluc O Conjugation Reduction Hydrolysis CYP3A4 CYP2C8 Carbamazepine N O NH2 Epoxide Hydrolase 10, 1111-epoxide OH OH N O NH2 Autoinduction CYP3A4 Inhibitors: Increased Carbamazepine Levels Erythromycin Clarithromycin  Troleandomycin  Propoxyphene (75%)  Diltiazem  Verapamil  Fluoxetine  Fluvoxamine Isoniazid Cimetidine  Loratadine  Itraconazole  Ketoconazole  Fluconazole  Miconazole  Grapefruit juice     CBZ-Diol Schachter SC. Exp Opin Investig Drugs. Drugs. 1999;8:11999;8:1-10. Carbamazepine Epoxide Carbamazepine T1/2 & Clearance  Antiepileptic and neurotoxic T1/2 (hours) Clearance (L/kg/hr) Single Dose 30 – 35 0.02 Monotherapy 12 0.064 8.6 Polytherapy 6–8 0.1 16.8  50% protein bound  CBZ:CBZE 0.1 – 0.25 Monotherapy (10 mg/L: 2.5 mg/L) 0.25 – 0.5 Polytherapy (10 mg/L: 5 mg/L) Dose (mg/kg)  Possible CBZE Toxicity > 0.35 ratio > 2 mg/L Carbamazepine Autoinduction Begins on first dose; complete on days 21 – 28 Dose Week 1 200 mg BID Carbamazepine Trough (mg/L) Day 1 Day 7 4 0 Carbamazepine Diurnal Fluctuations  Protein binding Metabolism Absorption (anticholinergic effects) – Michaelis-Menten absorption, 35% via zero order  Week 2 200 mg TID 4 1 40% with tid dosing; 75% with polytherapy Highest levels gradually increase throughout the day Consider highest dose HS Cannot always rely on one single level  Sustain released preparations minimize the toxicities of diurnal fluctuations  Week 3 200 mg QID 4 2 Week 4 300 mg TID 4 4 Carbamazepine Loading   PI suggests 200 mg bid increasing at weekly intervals by 200 mg/daily to tid - qid. Rationale: unconfirmed GI and CNS toxicity Loaded Carbamazepine Suspension (n=20) and tablets (n=20) 8 mg/kg if not on hepatic inducers; MD in 12 hours 10 mg/kg if on hepatic inducers; MD in 8 hours CBZ Susp: level of 4 mg/dL within 1 - 2 hours!  CBZ tablet: level of 4 mg/dL between 3 - 12 hrs Carbamazepine Extended Release Tegretol® XR   Administered every 12 hours Bioequivalent to IR q6h F = 70%  Osmotic release delivery system Single opening drilled on one side of tablet for drug release Casing is excreted in feces Increases in size Examine for chips & cracks   Cannot be crushed or chewed Cohen H, Howland MA, et al. Am J Health-Syst Pharm.1998;55:1134-40. Carbamazepine Extended Release Capsules (Carbatrol®)   Indicated for Seizures Three types of beads Immediate-Release Extended-Release Enteric-Release   Administered every 12 hours Bioequivalent to IR q6h  Cannot be chewed or crushed Can be opened and sprinkled on food F = 70%  Carbatrol® Vs Tegretol® XR Carbatrol® is bioequivalent to Tegretol® XR  Carbatrol® has less variability in the rate of absorption than Tegretol® XR  Carbatrol® may be opened and mixed with diluent and quickly administered through a feeding tube larger than 12 French  NS or Apple Juice did not clog the tube D5W and Sterile Water may clog the tube Use with 30 mL, and use 2 washings Case 1 80 yo, 80 kg, 6’, WM, to be placed on Carbamazepine. He is on no other medications. LD target = 10 mg/L & MD target = 8 mg/L LD = O Cytosolic Reduction (Cpp) (Vd) (S) (F) LD = Trileptal® Metabolic Pathway: No Epoxide, No Autoinduction (10 mg/L) [(1.4 L/Kg) (80 kg)] Gluc O No autoinduction Conjugation N N O O NH2 N NH2 O NH2 Oxcarbazepine (T1/2 = 2 hours) MHD (T1/2 = 9 hours) O Oxidation (1) (0.8) Loading Dose = 1400 mg Suspension for one dose Begin MD in 12 hours OH N O NH2 Hydrolysis CYP3A4 CYP2C8 Carbamazepine N O NH2 Epoxide Hydrolase 10, 1111-epoxide OH OH Autoinduction N O NH2 CBZ-Diol Schachter SC. Exp Opin Investig Drugs. Drugs. 1999;8:11999;8:1-10. Oxcarbazepine (Trileptal®)   Monotherapy or Adjunctive therapy for Partial Epilepsy 10-monohydroxy metabolite (MHD) Accounts for majority of efficacy MHD is almost completely renally eliminated Oxcarbazepine (Trileptal®)  BID dosing (Tab & Susp)  Suspension Tmax = 5 – 6 hours 300 mg/5mL Shale well May mix with water Use within 7 weeks after opening Bioequivalent to tablets – T1/2 increases to 19 hours; AUC doubles Qcr < 30 mL/minute – Initiate at half-dose, 300 mg daily in lieu of 300 mg bid Protein Binding is low at 40%  No adjustments necessary in mild to moderate hepatic disease Oxcarbazepine (Trileptal®)  Administered twice daily 300 mg bid; increased by 600 mg weekly to 600 mg bid Doses above 1200 mg/day are more effective esp. w/monoTx Max dose 2,400 mg/day Blood levels not necessary Devoid of hepatotoxicity and blood dyscrasias  Adverse effects VPA Indications and Plasma Levels Monotherapy for Simple & Complex Partial Seizures Monotherapy Mixed Seizures  Absence Seizures   Trough levels 50 – 100 mg/L Levels as high as 200 mg/L have been successfully utilized   SIADH CNS Gastrointestinal  Manic episodes with bipolar disorder  Prophylaxis of Migraine Headaches Trough levels 50 – 125 mg/L Css = 75 mg/L VPA Elimination  Metabolized almost 100% via liver 40% via mitochondrial β-oxidation Ω (Omega) oxidation 30 – 50% appears in the urine as a glucuronide conjugate Di-ene, 3-ene, 4-ene Metabolites CYP450 oxidation is minor pathway  Clearance  Adults on enzyme inducers & Pediatrics 8 mL/kg/hour (6 – 10 mL/kg/hour) 13 mL/kg/hour (10 – 15 mL/kg/hour) Valproate Capacity-Limited Protein Binding  Relationship between dose and plasm levels are nonlinear due to saturable protein binding  40 mg/L: 90% - 95% bound to albumin  50 - 70 mg/L: binding sites are saturated  130 mg/L: 82% bound  82% bound with renal failure  70% bound with Hepatic Cirrhosis Valproate Drug-Drug Interactions  Salicylates displace Valproate from protein binding sites Naproxen decreases VPA levels by 20% Valproate displaces warfarin  Valproate displaces diazepam  Inhibits diazepam and lorazepam metabolism Cimetidine inhibits VPA clearance  Erythromycin increases valproate levels  80 mcg/mL increased to 260 mcg/mL Clarithromycin and troleandomycin CYP450 Inducers Double the Clearance of VPA Carbamazepine Phenobarbital  Phenytoin  Primidone  Rifabutin  Rifampin    VPA is a weak inhibitor of CYP450 system Valproate Capacity-Limited Protein Binding Total 50 mcg/mL 100 mcg/mL 100 mcg/mL 150 mcg/mL 150 mcg/mL Percent Bound Bound 90% 90% 80% 90% 80% 45 mcg/mL 90 mcg/mL 80 mcg/mL 130 mcg/mL 120 mcg/mL Free 5 mcg/mL 10 mcg/mL 20 mcg/mL 20 mcg/mL 30 mcg/mL 200 mcg/mL 90% 180 mcg/mL 20 mcg/mL 200 mcg/mL 90% 160 mcg/mL 40 mcg/mL VPA Diurnal Fluctuations  Significant in the daytime  Minimal in the evening CYP450 fluctuations Protein binding fluctuations Free fatty acid fluctuations 1 g TID causing daytime drowsiness 1 g @ 8 AM, 500 mg @ 2 PM & 8 PM, 1 g HS  Use ER or sprinkles Valproic Acid Capsules (Depakene®)  Enteric Coated Dissociated to VPA in the small intestine  15% GI disturbances  Not sustained in its release 40% GI Disturbances  Take with food  Administer TID – QID Do not chew or open  Syrup  Divalproex Sodium Delayed-Release Tablets (Depakote®)  Irritates mouth and throat Delayed absorption 250 mg/5mL  Administer BID AED Inducers TID/QID Divalproex Sodium (Depakote®) Sprinkle Closely approximates a continuous infusion model  Open and sprinkle on food  Can swallow whole  Do not Chew Divalproex Sodium ExtendedRelease Tablets (Depakote® ER)   500 mg qd week 1 1 g qd week 2      Dose Conversion: Depakote to Depakote ER Depakote Total Daily Dose 500 – 625 750 – 825 1000 – 1125 1250 – 1375 1500 – 1625 1750 1875 – 2000 2125 – 2250 3000 - 3125 Indicated for migraines Depakote ER (mg) 750 1000 1250 1500 1750 2000 2250 2500 3500 Releases in gut, SI, LI over 18 – 24 hours 10 – 20% less level fluctuations that Depakote Cannot crush or chew May administer with food ER Tablet F = 0.9 Valproate Oral Loading Generally “not recommended” Successfully attempted in psychiatry  Empiric 15 mg/kg = Target 100 mg/L with LD Equation  Empiric 20 mg/kg = Target 140 mg/L with LD Equation  Day 1: 7.5 – 15 mg/kg  Day 2: 15 – 20 mg/kg  Empiric Dosing achieves a level of 100 – 140 mcg/mL  Administer as Depakote® NOT Depakene®   Empirically load a 70 kg patient at 15 mg/kg with oral valproate  10 mg/kg on Day 1 = 700 mg 250 mg TID  15 mg/kg on Day 2 = 1050 mg 250 mg QID  Slow Titration 10 mg/kg and increase by 5 – 10 mg/kg q2 – 4 days 250 mg TID on Day 1 followed by 250 mg QID on day 3,4, or 5 ? ? ? ? ? ? Questions Thanks! ? Questions ?