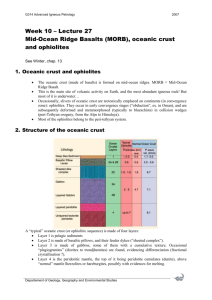
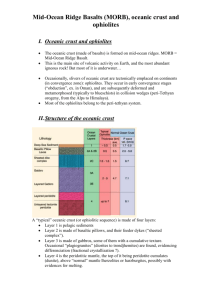
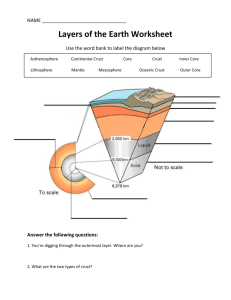
Recycling oceanic crust: Quantitative constraints
advertisement

Geochemistry Geophysics Geosystems 3 G Article Volume 4, Number 3 5 March 2003 8003, doi:10.1029/2001GC000223 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society ISSN: 1525-2027 Recycling oceanic crust: Quantitative constraints Andreas Stracke National High Magnetic Field Laboratory (NHMFL) and Department of Geological Sciences, Florida State University, 1800 East Paul Dirac Drive, Tallahassee, Florida 32306, USA. Department of Earth Sciences, Cambridge University, Downing Street, Cambridge CB2 3EQ, UK. Now at Max-Planck-Institute für Chemie, Abteilung Geochemie, J. J. Becher Weg 27, 55128 Mainz, Germany.(stracke@mpch-mainz.mpg.de) Michael Bizimis and Vincent J. M. Salters National High Magnetic Field Laboratory (NHMFL) and Department of Geological Sciences, Florida State University, 1800 East Paul Dirac Drive, Tallahassee, Florida 32306, USA (bizimis@magnet.fsu.edu; salters@magnet.fsu.edu) [1] Recycled ancient oceanic crust with variable amounts of aging, or inclusion of sediments of differing types and origins has often been invoked as a source for present-day ocean island basalts (OIB), but the current evidence remains largely qualitative. Previous quantitative modeling has shown that much has to be learned in order to better understand the implications of crustal recycling on mantle heterogeneity. Here, we present new model calculations incorporating recent constraints on subduction-zone processes and the composition of subducted sediments. Modeled compositions of the recycled oceanic crust vary widely as a function of the recycling age and composition of the oceanic crust. HIMU-type sources can only be created by recycling igneous oceanic crust if it has undergone substantial modification during subduction. Although the required modifications are qualitatively consistent with dehydration processes in subduction zones, the many uncertainties prevent a precise estimate of the isotopic composition of ancient recycled igneous crust. Inclusion of sediments increases the isotopic variability and although the resulting Sr and Nd isotopic signatures can be similar to enriched mantle (EM) signatures, the Pb isotopic composition of EMtype OIB is difficult to reconcile with the presence of sediment in their sources. The large variability of modeled compositions of the subducted crust suggests that if mantle heterogeneity is largely formed by crustal recycling, each OIB is likely to have a unique isotopic composition resulting from specific combinations of composition, age and subduction modification of the subducted crust. Given the variability of the recycled components, a small number of relatively well-defined enriched compositions can only be explained if either the subduction processing of oceanic crust is a far better defined process than observation would seem to indicate, or, the intramantle disaggregation and mixing of compositionally diverse recycled materials is surprisingly efficient. Components: 19,603 words, 8 figures, 5 tables. Keywords: Recycling; oceanic crust; isotope geochemistry; trace elements; isotopes. Index Terms: 1025 Geochemistry: Composition of the mantle; 1040 Geochemistry: Isotopic composition/chemistry; 1065 Geochemistry: Trace elements (3670); 3640 Mineralogy and Petrology: Igneous petrology. Received 30 August 2001; Revised 4 September 2002; Accepted 4 October 2002; Published 5 March 2003. Stracke, A., M. Bizimis, and V. J. M. Salters, Recycling oceanic crust: Quantitative constraints, Geochem. Geophys. Geosyst., 4(3), 8003, doi:10.1029/2001GC000223, 2003. Copyright 2003 by the American Geophysical Union 1 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust 1. Introduction [2] The potential importance of recycled oceanic crust to contribute to mantle heterogeneity was first recognized by Armstrong [1968]. Subsequently, Chase [1981] and Hofmann and White [1982] have invoked recycled oceanic crust to be a source for ocean island basalts (OIB; see references given below). [3] While recycling of oceanic crust into the mantle is an integral part of plate tectonics, exactly how this recycling process manifests itself in the chemical composition of OIB is still a debated issue and is key in understanding the chemical evolution of the Earth’s mantle-crust system. Over Earth’s history, recycled oceanic crust amounts to roughly 8–10% of the mass of the mantle (assuming a production rate of about 3km2/year [Hofmann and White, 1982], an average thickness of the oceanic crust of about 7km [White et al., 1992]; density of the oceanic crust = 3.3g/cm3, age of the Earth = 4.55*109 years, mass of the mantle = 4*1024 kg). Depending on the exact modus of plate tectonics, there might be considerable uncertainty in this estimate, but it confirms that recycling of oceanic crust may play a significant role in assessing the chemical composition of OIB and in understanding mantle-crust evolution. [4] White [1985] and Zindler and Hart [1986] classified OIBs into three isotopically distinct groups (e.g., HIMU = high m = 238U/204Pb, EMI and EMII, EM = enriched mantle). Although a variety of mechanisms have been proposed to cause these enriched isotopic signatures in OIB, recycling of oceanic crust with variable amounts of aging, modification during seafloor alteration and subduction, or inclusion of sediments of differing types and origins, has been invoked most often. The HIMU-type OIB have been explained by recycling pure igneous oceanic crust [e.g., Chauvel et al., 1992, 1997; Halliday et al., 1988; Hart, 1988; Hauri and Hart, 1993; Hauri et al., 1996; Hofmann, 1997; Lassiter and Hauri, 1998; Nakamura and Tatsumoto, 1988; Palacz and Saunders, 1986; Reisberg et al., 1993; Roy-Barman and Allègre, 1995; Salters and White, 1998; 10.1029/2001GC000223 Vidal et al., 1984; Weaver, 1991; Zindler and Hart, 1986]. The EM-type OIB have been explained by recycling oceanic crust plus minor amounts of sediment with different composition [e.g., Barling and Goldstein, 1990; Blichert-Toft et al., 1999; Chauvel et al., 1992; Eisele et al., 2002; Hart, 1988; Hauri and Hart, 1993; Hauri et al., 1996; Hemond et al., 1994; Hofmann, 1997; Le Roex et al., 1990; Rehkämper and Hofmann, 1997; RoyBarman and Allègre, 1995; Weaver, 1991; Weis et al., 1993; White and Duncan, 1995; Woodhead and Devey, 1993; Woodhead and McCulloch, 1989; Wright and White, 1987; Zindler and Hart, 1986]. [5] An assumption implied in most of these studies is that recycling of oceanic crust leads to the limited number of enriched isotopic components in the mantle (HIMU, EMI and EMII) and that mixing between these end-members or components can explain most of the isotopic variability in OIB [Zindler and Hart, 1986]. However, this is only one possible ‘‘picture’’ of the isotopic variability in the mantle. Independent of the way in which the chemical heterogeneities are introduced into the mantle, two end-member scenarios are possible to explain the isotopic variability of OIB sources: (1) There are few different enriched compositions in the mantle (HIMU, EMI and EMII) and the isotopic signatures in each OIB are produced by unique mixtures between these same end-member compositions, or, (2) each OIB-source has its own unique composition, i.e. there are at least as many end-members as OIBs. [6] The model calculations presented here allow for a wide variety of compositions of the ancient recycled crust (both in terms of its initial isotopic and trace element composition), recycling ages, alteration during subduction and relative quantities of the recycled materials (basalt and sediment). The model calculations and input parameters are supplied via Microsoft Excel spread sheets, see auxilliary material available at http://www.g-cubed.org. The reader is encouraged to use these spreadsheets and use her/his own input parameters to explore possible results. Based on our results of the modeling we find that the isotopic composition of 2 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust ancient recycled crust varies considerably as a function of composition, age and subduction modification. By simple modeling as presented here, it remains difficult to find suitable combinations of parameters that yield isotopic compositions of either HIMU or EM-type basalts. 2. Quantifying the Process of Recycling Oceanic Crust [7] In order to calculate the radiogenic isotope composition of the bulk subducted crust, its age and initial isotopic composition have to be known, as well as its parent-daughter (P/D) ratio for each isotopic system. A detailed look at the processes involved in recycling oceanic crust, however, shows that estimating these three parameters involves many uncertainties and that it is difficult to uniquely define the final composition and age of the subducted crust. In the following, the term ‘‘oceanic crust’’ is used for crust not yet modified by subduction zone processes, whereas the term ‘‘subducted crust’’ is used for crust that has been modified during subduction. [8] Recycling starts with the formation and hydrothermal alteration of the oceanic crust at mid ocean ridges (MOR), followed by the progressive deposition of oceanic sediments with increasing age of the crust. As it enters a subduction zone, the bulk oceanic crust (igneous crust + sediment) loses parts of its sedimentary cover which is scraped off and accumulated in the accretionary prism. Finally, during subduction, the subducted crust undergoes a series of phase changes and different parts of it are dehydrated and/or melted to different extents [e.g., Bizimis et al., 2000; Elliott et al., 1997; Tatsumi, 1989; Tatsumi and Kogiso, 1997; Turner and Hawkesworth, 1997], before the slab is further physically and mineralogically modified during transport into the deeper mantle [e.g., Grand et al., 1997; van der Hilst et al., 1997]. [9] For quantitative modeling, we need to have a representative estimate of the composition of the bulk oceanic crust entering the subduction zone and need to quantify the geochemical processes that 10.1029/2001GC000223 modify the package during subduction. The composition of average fresh igneous crust is well known [e.g., Hofmann, 1988; Sun and McDonough, 1989] and the composition of the hydrothermally altered igneous crust can be directly measured at some distance from the ridge [Staudigel et al., 1995, 1996]. Plank and Langmuir [1998] provide an estimate of the composition and variability of sediments overlying the igneous crust, so that the input compositions are relatively well characterized. The extent of alteration during subduction is more difficult to estimate. A number of recent studies which investigate sub-arc processes on a quantitative basis will be used in this study [e.g., Ayers, 1998; Ayers et al., 1997; Brenan et al., 1995; Johnson and Plank, 2000; Kogiso et al., 1997; McCulloch and Gamble, 1991; Stalder et al., 1998]. 3. Constraints From Quantitative Recycling Models 3.1. Recycling Igneous Oceanic Crust 3.1.1. Modeling the Composition of the Igneous Oceanic Crust [10] Figure 1 shows the calculated isotopic compositions for ancient fresh and altered basalts [Hofmann, 1988; Staudigel et al., 1995, 1996; Sun and McDonough, 1989], and oceanic gabbros [Hart et al., 1999; Zimmer et al., 1995]. The details of the calculations are given in the Figure caption and by using the spread sheet ‘‘basalt + sediment_recycling’’, the calculations can be reproduced and different compositions can be investigated. Also, an inverse approach similar to that of Hauri and Hart [1993] is undertaken in order to calculate the trace element composition of the recycled oceanic crust which is necessary to yield HIMU isotopic signatures (see Figure 2, Table 2, and spread sheet ‘‘P/D_HIMUsource’’). In both cases, the calculations are done for varying recycling ages, i.e. varying initial isotopic composition of the oceanic crust (the recycling age is the age at which the recycled crust started to evolve as a closed system until present-day melting). The initial isotopic composition of the 3 of 33 3 Geochemistry Geophysics Geosystems G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 0.5134 MORB altered MORB, Staudigel et al., 1995 0.5132 0.5Ga 143Nd/144Nd 0.5130 1Ga 1Ga 1Ga 0.5128 HIMU EMII 2Ga 0.5126 N-MORB Hofmann, 1988 2Ga 0.5124 EMI average 735B Gabbro Hart et al., 1999 0.5122 initial 87Sr/86Sr affected by seawater alteration no seawater alteration 0.5120 0.702 0.703 0.704 0.705 0.706 a 0.707 0.708 87Sr/86Sr 42 41 HIMU EMII 208 Pb/ 204 Pb 40 EMI 39 38 MORB 37 1Ga 0.5Ga 2Ga 1Ga 36 b 35 15 16 17 18 19 20 21 22 23 24 206 Pb/ 204 Pb Figure 1. Shown is the calculated present-day-isotopic composition of ancient recycled oceanic crust as a function of recycling age (2Ga – 0.5Ga) in a variety of isotope ratio diagrams. Although the Nd and Hf isotope composition of recycled oceanic crust can be similar to those of HIMU basalts (Figure 1e), none of the compositions used here (average N-MORB [Hofmann, 1988], oceanic gabbro [Hart et al., 1999], and average altered MORB [Staudigel et al., 1995, 1996]; see also Table 1) develops 208Pb/204Pb similar to those in OIB (Figure 1b). This indicates that especially the U-Th-Pb budget of the recycled oceanic crust has to be substantially modified during subduction if the HIMU source is assumed to originate from recycled oceanic crust. See section 3.1 for detailed discussion, and appendix B and auxiliary material and spread sheet ‘‘basalt + sed_recycling’’ for details of the calculations. Plotted data for EMI-type basalts are from the Tristan da Cunha, Pitcairn, and Kerguelen islands and the Walvis ridge, EMII basalts are from the Society, Samoa, and Marquesas islands and HIMU-type basalts are from St. Helena, and the Tubaii, Rurutu, Mangaiia, and Rimatara islands, MORB data are from the Atlantic, Pacific and Indian ocean ridges. For data and references see spread sheet ‘‘basalt + sed_recycling’’. 4 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 0.5134 MORB 0.5132 143 Nd/ 144 Nd 0.5Ga 0.5130 1Ga HIMU 1Ga 0.5128 2Ga 0.5126 EMII 2Ga 0.5124 EMI 0.5122 c 0.5120 15 16 17 18 19 20 21 22 23 24 206 Pb/ 204 Pb 0.708 EMII 0.707 EMI 87Sr/86Sr 0.706 0.705 0.5Ga 0.704 1Ga 2Ga 0.703 2Ga 2Ga 1Ga HIMU 1Ga 2Ga 1Ga d MORB 0.702 15 16 17 18 19 20 21 22 23 24 206 Pb/ 204 Pb Figure 1. (continued) oceanic crust is also a function of the assumed differentiation age of the mid ocean ridge basalt (MORB) source (the differentiation age of the MORB source is the age at which the MORB source was derived from the Bulk Earth (BE) reservoir, and is referred to as source age in the following; source age = 2 Ga in Figure 1). For details of the modeling and the isotopic evolution of the MORB source see appendix B and the spread sheets ‘‘basalt + sediment_recycling’’ and ‘‘P/D_HIMUsource’’. 3.1.2. Isotopic Composition of Ancient Igneous Oceanic Crust [11] Due to their high U/Pb ratios, ancient seawater altered basalts have 206Pb/204Pb ratios that are far more radiogenic than those of any OIB (recycling ages >1Ga; see Figures 1b–1d). Owing to their 5 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 0.2836 MORB 176Hf/177Hf 0.2834 0.2832 EMII 0.2830 1Ga 0.2828 1Ga 1Ga HIMU EMI 2Ga 0.2826 2Ga 2Ga e 0.2824 0.2822 0.5120 0.5122 0.5124 0.5126 0.5128 0.5130 0.5132 0.5134 143 Nd/ 144 Nd Figure 1. (continued) high Rb/Sr ratios, ancient altered basalts also develop high 87Sr/86Sr ratios, which are unlike the low 87Sr/86Sr values of HIMU basalts (Figures 1a, 1d; see also Hart and Staudigel [1989]). Thus, the Sr and Pb isotope composition of ancient altered MORB preclude, rather than support the contention that ancient altered crust yields HIMU compositions [Hart and Staudigel, 1989]. Also, any endmember with such radiogenic Sr and Pb isotope composition would produce mixing trends oblique to the OIB arrays in Sr-Nd-Pb isotope diagrams (Figure 1), which are not readily apparent. [12] Hauri and Hart [1993] compared calculated P/ D-ratios of the HIMU source with the range of P/D ratios in modern MORB. They calculated a range of Rb/Sr, Sm/Nd and Lu/Hf ratios for a range of ages and present-day isotopic compositions of the HIMU source, and derived a range of U/Pb, Th/Pb, and Th/ U ratios from the slope of the tie lines between the MORB and HIMU fields in Pb-Pb isotope diagrams. Hauri and Hart [1993] noted that their inferred range of P/D ratios of the HIMU source is similar to the range in fresh basalts and argued that the alteration signature is removed during subduction and/or that the altered oceanic crust only constitutes a small portion of the recycled crust. In this study, we have calculated P/D ratios of the HIMU source for recycling/source ages between 3 and 0.5 Ga and a range of isotopic compositions for the present-day HIMU source (see Tables 1 and 2, Figure 2, and spread sheet ‘‘P/D_HIMUsource’’). The calculated range of P/D ratios for the HIMU source agrees well with the range calculated by Hauri and Hart [1993], except for Th/U. The range of Th/U ratios calculated from the Pb-Pb isotope systematics by Hauri and Hart [1993] extends to lower values (1.4–3.5) compared to our inversion calculations (2.9–3.7; Table 1, Figure 2). [ 13 ] 87 Figure 2 shows the range of calculated Rb/86Sr, 147Sm/144Nd, 176Lu/177Hf, 238U/204Pb (m),232Th/238U (k), and 232Th/204Pb (w) ratios for the HIMU source for recycling/source ages of 3–0.5 Ga (gray field in Figure 2; see also Tables 1, 2, and spread sheet ‘‘P/D_HIMUsource’’). The broad overlap between the range of 87Rb/86Sr, 147Sm/144Nd, and 176Lu/177Hf in fresh MORB and the HIMU source in Figure 2 suggests that, to a first approximation, the Sr, Nd and Hf isotope signatures of HIMU basalts can be explained by ancient recycled fresh MORB (see also Figures 1a, 1d, and 1e). However, the 8 7 Rb/ 8 6 Sr, 1 4 7 Sm/ 1 4 4 Nd and 176 Lu/177Hf ratios required for the HIMU source depend strongly on the assumed recycling/source age (i.e. its initial isotopic composition). For any 6 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 100 Frequency up to 61 12 60 8 40 4 20 0 0 0.10 0.14 0.18 0.22 0.26 0.30 0 4 8 147Sm/144Nd 12 16 20 24 28 32 36 40 238U/204Pb 40 80 e b Frequency d altered MORB = 47.8 16 a 60 30 40 20 20 10 0 up to 177 0 0.004 0.010 0.016 0.022 0.028 0.034 0.040 0.046 0 176Lu/177Hf 10 20 30 40 50 60 70 80 90 100 232Th/204Pb 20 c 16 60 12 40 8 20 4 f altered MORB = 0.36 Frequency 80 0 0 0 0.02 0.06 0.10 0.14 0.18 0.22 0.26 0.30 87Rb/86Sr 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 232Th/238U Average MORB glasses, this study N-MORB, Hofmann, 1988 average Gabbro, Hart et al., 1999 altered MORB, Staudigel et al., 1996 bulk crust range for the HIMU source recycling/source age = 0.5-3Ga range for the HIMU source recycling, source age = 2Ga Figure 2. Histograms showing the variation and frequency of parent-daughter (P/D) ratios in present-day MORB glasses (compiled from the LDEO petrological database at http://petdb.ldeo.columbia.edu/petdb; see spread sheet ‘‘P/D_data_MORB’’) compared to the range of P/D ratios calculated for the HIMU source (shaded field). Calculations assume a range of source ages of 3 – 1Ga and a range of recycling ages of 3 – 0.5Ga (gray field). The stippled field is the range of P/D ratios for a 2Ga old HIMU source (recycling = source age = 2Ga). Also shown are the average of the compiled ratios (average MORB, this study), as well as the average N-MORB of Hofmann [1988], altered MORB [Staudigel et al., 1996], gabbro; [Hart et al., 1999] and our estimate of the bulk igneous crust (see also Tables 1 and 3). specific combination of recycling and source ages, the range in P/D ratios is narrower than the total range for recycling/source ages between 3 and 0.5 Ga (compare the stippled field for a recycling/source age of 2 Ga with the gray field for recycling/source ages between 3 and 0.5 Ga in Figure 2; compare also Figure 2 with Tables 1 and 2). [14] In general, most fresh MORBs have 87Rb/86Sr <0.07, 147Sm/144Nd between 0.19 and 0.22, and 7 of 33 3 Geochemistry Geophysics Geosystems G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust Table 1. Parent-Daughter (P/D) Ratios in Modern Fresh and Altered MORB and Oceanic Gabbros, as Well as P/D Ratios Calculated for Recycled Oceanic Crust in Order to Yield HIMU Signaturesa 87 Rb/86Sr 147 Sm/144Nd 176 Lu/177Hf 238 U/204Pb Total range calculated for recycled oceanic crust (HIMU source, recycling ages 0.5 – 3Ga) 0.012 – 0.121 0.103 – 0.252 0.007 – 0.039 12 – 61 Range in modern MORB glasses (LDEO petrological database) 0.003 – 0.275 0.116 – 0.292 0.009 – 0.044 3 – 35 Average modern MORB glasses (LDEO petrological database) 0.053 ± 40 0.201 ± 30 0.024 ± 5 12 ± 5.6 N-MORB Hofmann [1988] 0.032 0.203 0.028 9.1 N-MORB Sun and McDonough [1989] 0.018 0.218 0.032 9.8 Altered MORB, Staudigel et al. [1996] 0.241 0.228 0.031 47.8 Average gabbro, Gabal Gerf ophiolithe, Zimmer et al. [1995] 0.006 0.169 0.023 5.1 Average gabbro, 735B, Hart et al. [1999] 0.006 0.183 0.027 4.5 Bulk oceanic crust (25% altered MORB + 25% N-MORB + 50% gabbro; [Hart et al., 1999; Hofmann, 1988; Staudigel et al., 1996]) 0.064 0.197 0.028 14.8 232 Th/204Pb 232 Th/238U 45 – 177 2.9 – 3.7 7 – 93 1.5 – 5.3 33 ± 18 2.75 ± 0.5 24.6 2.7 25.8 2.6 17.3 0.36 8.9 1.8 8.2 1.8 19 1.3 a For the trace element data in modern MORB glasses see spread sheet ‘‘P/D_data_MORB’’ (data are compiled from the Lamont Doherty Earth Observatory petrological database at http://petdb.ldeo.columbia.edu/petdb). 176 Lu/177Hf between 0.020 and 0.028 (Figures 2a– 2c) so that HIMU isotopic signatures only result for recycling ages roughly >1.5 Ga for Sr and Nd isotopes and <1.5 Ga for Hf isotopes (compare Figure 2 with Tables 1 and 2). If the initial Sr isotope composition of the igneous crust is modified by seawater alteration, lower 87Rb/86Sr are required than in case of pristine initial 87Sr/86Sr ratios (Figure 1a). In contrast to the 87Rb/86Sr, 147 Sm/144Nd, and 176Lu/177Hf ratios, the calculated Table 2. Calculated Parent-Daughter (P/D) Ratios in Recycled Oceanic Crust in Order to Produce HIMU-Signatures as a Function of the Recycling and Source Age of the MORB-Sourcea Recycling age 0.5Ga 1Ga 1.5Ga 2Ga 2.5Ga 3Ga 0.037 – 0.054 0.201 – 0.213 0.032 – 0.036 0.037 – 0.051 0.205 – 0.215 0.034 – 0.037 13.0 – 17.2 3.4 – 3.7 48 – 59 12.1 – 15.4 3.5 – 3.7 45 – 54 Source Age of the MORB Source: 1Ga 87 Rb/86Sr 147 Sm/144Nd 176 Lu/177Hf 0.160 – 0.221 0.005 – 0.025 0.221 – 0.252 0.034 – 0.044 87 Rb/86Sr 147 Sm/144Nd 176 Lu/177Hf 0.010 – 0.094 0.117 – 0.178 up to 0.011 Source Age 0.010 – 0.052 0.178 – 0.209 0.019 – 0.030 of the MORB Source: 2Ga 0.010 – 0.038 0.010 – 0.031 0.199 – 0.219 0.209 – 0.224 0.029 – 0.036 0.034 – 0.039 87 Rb/86Sr 147 Sm/144Nd 176 Lu/177Hf 0.037 – 0.121 0.103 – 0.164 up to 0.007 Source Age 0.037 – 0.079 0.164 – 0.195 0.015 – 0.025 of the MORB Source: 3Ga 0.037 – 0.065 0.037 – 0.058 0.185 – 0.205 0.195 – 0.210 0.024 – 0.031 0.029 – 0.034 238 36.5 – 61.3 2.9 – 3.2 117 – 177 21.8 – 33.7 3.1 – 3.4 74 – 103 U/204Pb 232 Th/238U 232 Th/204Pb Pb Evolution: Single Stage 16.9 – 24.6 14.5 – 20.0 3.17 – 3.5 3.3 – 3.6 59 – 79 52 – 66 a The range of the isotopic composition of the HIMU source is: 87Sr/86Sr = 0.7027 – 0.7033; 143Nd/144Nd = 0.5128 – 0.5130; 176Hf/177Hf = 0.2828 – 0.2830; 206Pb/204Pb = 20 – 22; 208Pb/204Pb = 39.5 – 41. The isotopic evolution of the MORB reservoir is described in detail in appendix B, see spread sheets ‘‘basalt + sed_recycling’’ and ‘‘P/D_HIMUsource’’ and auxiliary material for details of the calculations. 8 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust m (238U/204Pb), k (232Th/238U), and w (232Th/204Pb) in the HIMU source overlap those of fresh MORB only to a small degree (Figures 2d–2f; Tables 1 and 2; [Hart and Staudigel, 1989; Hauri and Hart, 1993]). Only the highest m and w values observed in fresh MORB would be suitable for the HIMU source, and only for recycling ages >1.5–2 Ga (compare Table 2 and Figure 2), but most MORB have m and w that are too low for the HIMU source (see distribution in Figures 2d and 2e). High m correspond to high w values in MORB, but neither m nor w correlate with k. The k of the HIMU source must be higher than in most modern MORB (Figure 2f ). Therefore, the HIMU source must be depleted in Pb relative to Th and U, but also depleted in U relative to Th or enriched in Th relative to U compared to modern MORB. [ 15 ] 176 Although oceanic gabbros have similar Lu/177Hf ratios than MORB, they have lower 87 Rb/86Sr, m, k, and w and also slightly lower 147 Sm/144Nd ratios, based on the two estimates of average gabbro compositions given in Table 1 [Hart et al., 1999; Zimmer et al., 1995] (Figures 2a–2f). Due to their lower m and k values (lower than those of BE), ancient oceanic gabbros develop even more unradiogenic Pb isotope ratios than the basalts (206Pb/204Pb < N-MORB, Figures 1b–1d, Table 1; [Hart et al., 1999]). The gabbros also develop slightly lower Sr and Nd isotopic composition than the basalts. The 143Nd/144Nd ratios of the gabbros tend to be too low for HIMU basalts, at least for recycling ages >1 Ga (Figures 1a, 1c, and 1e, Table 2). [16] In summary, although some trace element ratios in altered or fresh oceanic basalts and gabbros are suitable for the HIMU source, the low w in all these rocks lead to 208Pb/204Pb ratios that are lower than the 208Pb/204Pb ratios in most OIB, but especially HIMU-type basalts (see Figures 1b, 2, Tables 1 and 2). This conclusion is almost independent of the assumed recycling/source age. Therefore, removal of the alteration signature during subduction [Hauri and Hart, 1993] is insufficient to transform recycled oceanic crust into appropriate OIB and especially HIMU sources. The fact that all the components of the oceanic crust, altered and fresh basalts, and oceanic gab- 10.1029/2001GC000223 bros, have low w also indicates that any mixture between these components cannot be a suitable source for OIB without further modification during subduction. 3.1.3. Effects of Subduction-Zone Processing on Igneous Oceanic Crust [17] Oceanic crust is modified during sub-arc processing by preferential extraction of some elements via fluid-basalt interaction. In the following, it is investigated whether the resulting subducted crust can be a suitable source of OIB, and HIMU-type basalts in particular. [18] We use four examples for input compositions of the oceanic crust: the N-MORB of Hofmann [1988], the average oceanic gabbro of Hart et al. [1999] and the altered crust of Staudigel et al. [1995, 1996] (Pb in the altered crust is estimated by assuming Ce/Pb = 25). In addition, we estimate an average composition of the bulk igneous crust (basalt and gabbros) by mixing basalt and gabbros in equal proportion and assuming that the basaltic portion consists to equal parts of altered and fresh oceanic crust (bulk crust = 25% fresh N-MORB + 25% altered MORB + 50% gabbro; Tables 1 and 3). It is then calculated how the P/D ratios of the oceanic crust have to change during subduction in order to transform the oceanic crust into appropriate HIMU sources (assuming an age of 2Ga) and whether the necessary changes are in agreement with current constraints on sub-arc alteration mechanisms [e.g., Ayers, 1998; Ayers et al., 1997; Brenan et al., 1995; Kogiso et al., 1997; McCulloch and Gamble, 1991; Stalder et al., 1998]. The P/D ratios of all four compositions are given in Table 1 and Figure 2. For comparison Figure 2 also shows the range of P/D ratios for a 2 Ga old HIMU source (recycling/source age = 2Ga, stippled field in Figure 2). 3.1.3.1. Rb-Sr System [19] Comparing the 87Rb/86Sr ratios of all four examples (N-MORB, gabbro, altered crust, and bulk crust) with the range of 87Rb/86Sr ratios for a 2 Ga old HIMU source (Figure 2c) shows that except for the gabbros, the 87Rb/86Sr ratios have 9 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust Table 3. Estimates of Trace Element Mobility During Sub-Arc Magmatism Used in Figures 1, 3, 4, and 8a Cs Rb Ba Th U Nb Ta La Ce Pb Nd Sr Zr Hf Sm Eu Ti Gd Dy Y Er Yb Lu Bulk Igneous Crust Mobility Bulk Subducted Igneous Crust GLOSS Mobility Fluid-Sediment Mobility Melt-Sediment Sub-Arc Modified GLOSS (sed-melt) 0.051 2.99 13.87 0.142 0.115 2.03 0.129 3.83 11.95 0.48 9.55 136 82 2.28 3.11 1.13 8212 4.24 5.19 29.1 3.14 3.03 0.45 51% 81% 53% 38% 76% 4% 4% 56% 51% 81% 22% 41% 22% 22% 14% 8% 6% 5% 4% 2% 1% 1% 1% 0.025 0.57 6.59 0.088 0.027 1.95 0.124 1.68 5.89 0.09 7.45 81 64 1.78 2.69 1.04 7735 4.03 5.01 28.5 3.13 2.99 0.45 3.48 57.2 776 6.91 1.68 8.94 0.63 28.8 57.3 19.9 27 327 130 4.06 5.78 1.31 3716 5.26 4.99 29.8 2.92 2.76 0.41 28% 40% 44% 15% 17% 21% 22% 33% 35% 24% 37% 38% 10% 11% 33% 33% 29% 28% 23% 20% 19% 17% 16% 43% 34% 32% 31% 31% 26% 27% 24% 23% 13% 21% 46% 47% 45% 20% 20% 24% 19% 19% 19% 19% 19% 20% 1.97 37.8 528 4.76 1.17 6.65 0.46 21.8 44.4 17.2 21.4 178 69 2.23 4.60 1.04 2826 4.25 4.04 24.2 2.35 2.24 0.33 a The bulk oceanic crust is derived by mixing 25% altered MORB [Staudigel et al., 1996] + 25%N-MORB [Hofmann, 1988] + 50%gabbro; [Hart et al., 1999]. GLOSS is the ‘‘global subducted sediment’’ as given by Plank and Langmuir [1998]. Mobility estimates for sub-arc basalt alteration are taken from Kogiso et al. [1997] but are modified to acquire HIMU isotopic signatures for a recycling age of 2Ga. Mobility estimates for fluidsediment exchange at 700C and melt-sediment exchange at 900C are derived according to the parameters given by Johnson and Plank [2000] (their Table 1 and 8, formula for mobility as given in their paragraph 38). to decrease. In case of the fresh basalts (NMORB), however, it is clear from their overall compositional variability (Figure 2c and Tables 1 and 2) that the required amount of Rb loss decreases with decreasing 87Rb/86Sr of the basalt. Some fresh basalts with the lowest 87Rb/86Sr observed even require a slight increase in 87Rb/ 86 Sr (Figure 2c). The required decrease in 87Rb/ 86 Sr for the altered crust has to be an order of magnitude higher than for the fresh basalts (Figure 2c; Table 1). The necessary Rb loss has to be even more severe in case the initial Sr isotopic composition is raised by seawater alteration (see Figure 1a). As a consequence, the Rb gained during alteration (no Sr is gained [Staudigel et al., 1995, 1996]) has to be removed almost quantitatively from the altered oceanic crust during subduction. [20] Note that the discussion above is valid only for a recycling and source age of 2Ga. For a given source age, calculated 87Rb/86Sr ratios of the HIMU source increase with decreasing recycling age (decreasing initial isotopic composition). For a given recycling age, 87Rb/86Sr ratios of the HIMU source increase with increasing source age (decreasing initial isotopic composition; see Table 2). Therefore, required changes in 87Rb/86Sr ratios of the oceanic crust are systematically different as a function of recycling/source age. However, relatively old source ages (>1.7 Ga) are required based on the Sr isotope evolution of the Bulk Earth (BE) and the present-day isotopic composition of MORB, because the initial 87 Sr/86Sr ratio of the BE reservoir becomes greater than that of present-day MORB roughly between 1.7 and 2.2 Ga ago (for present-day MORB values of 0.7020 and 0.7027 and present-day BE of 0.7050). [21] Rb partitions preferentially into subduction zone fluids compared to Sr [e.g., Ayers, 1998; Ayers et al., 1997; Brenan et al., 1995; Keppler, 1996; Kogiso et al., 1997; McCulloch and Gamble, 1991]. Estimates of bulk fluid-slab partition coefficient ratios (DRb/DSr), a measure of the relative 10 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust mobility of Rb and Sr, differ significantly: DRb/DSr = 0.2 [Ayers, 1998], DRb/DSr = 0.5 [McCulloch and Gamble, 1991]). With the given partition coefficients for Dslab/fluid [Ayers, 1998; Brenan et al., 1995; McCulloch and Gamble, 1991; Stalder et al., 1998] and assuming the fraction of the fluid interacting with the oceanic crust is 5%, the calculated Rb loss is between 26 and 49% and the Sr loss is between 3 and 30%. The Rb and Sr loss increases proportionally with the amount of fluid. Kogiso et al. [1997] estimate a Rb and Sr loss of 63% and 41%, respectively. Thus, sub-arc alteration lowers the 87Rb/86Sr ratios of the oceanic crust, but due to the range of Dslab/fluid and the dependence on the amount of fluid interacting with the oceanic crust, the exact magnitude of this effect is uncertain. [22] Note that for all the studies considered, the amount of Rb loss relative to Sr loss is insufficient to account for the required Rb loss (relative to Sr) of the altered crust, indicating that altered crust alone cannot be an appropriate HIMU source, even after modification during subduction. This conclusion is independent of the assumed recycling and source age. For the bulk igneous crust, the Rb budget is dominated by the altered crust, so that the necessary amount of Rb loss depends critically on the proportion of the altered crust (see Figure 2c). For the bulk crust composition considered here, approximately three times as much Rb compared to Sr has to be removed in order to get 87Rb/86Sr ratios similar to those of the HIMU source (source and recycling age = 2Ga), which is within the range of the experimental estimates of Rb and Sr mobility. 3.1.3.2. Sm-Nd and Lu-Hf Systems [23] Overall, for the four examples considered, 147 Sm/ 144 Nd ratios are close to the required 147 Sm/144Nd ratios of a 2 Ga old HIMU source (Figure 2a, and compare Tables 1 and 2). The 147 Sm/144Nd ratios of the basalt, gabbro, and the bulk crust have to increase, while the 147Sm/144Nd ratios of the altered oceanic crust have to decrease slightly (Figure 2a). The 176Lu/177Hf ratios of all four compositions (fresh or altered basalts, gabbros, and bulk crust) have to increase during subduction (Figure 2b, Tables 1 and 2). 10.1029/2001GC000223 [24] As for Rb and Sr, however, the necessary changes in 147Sm/144Nd and 176Lu/177Hf ratios are dependent on the assumed recycling/source age. 147Sm/144Nd and 176Lu/177Hf ratios have to decrease with decreasing recycling ages (for any given source age). For any given recycling age, older source ages require lower 147Sm/144Nd and 176 Lu/177Hf ratios in the subducted crust to be suitable HIMU sources (Figure 1e, Tables 1, and 2; see also Salters and White [1998]). Since the 147 Sm/144Nd and 176Lu/177Hf ratios of the oceanic crust are not expected to change significantly during hydrothermal alteration, the range of 147 Sm/144Nd and 176Lu/177Hf ratios of the fresh oceanic crust and gabbros (Tables 1 and 2, Figures 2a, 2b) should be representative of the oceanic crust in general. Based on the most abundant 147 Sm/144Nd and 176Lu/177Hf ratios in MORB (0.19 147Sm/144Nd 0.22; 0.020 176Lu/177Hf 0.028; see distribution in Figures 2a and 2b), the 147 Sm/144Nd ratios of the oceanic crust are within the range of appropriate values for the HIMU source only for recycling ages 1.5 Ga, while 176 Lu/177Hf ratios are within the range of HIMU values only for recycling ages 1.5 Ga (compare Tables 1 and 2). [25] Unfortunately, few direct studies of the behavior of Lu and Hf during subduction processes are available, so that their behavior is inferred from that of chemically similar elements (other heavy rare earth elements (HREE, e.g., Yb) or Y and Zr, respectively). The behavior of the high field strength elements (HFSE) Zr and Hf depends strongly on the amount of rutile in the subducted slab [Ayers, 1998; Brenan et al., 1994, 1995; McCulloch and Gamble, 1991; Stalder et al., 1998]. HFSE are very efficiently retained by rutile, so that even small amounts of rutile (<1%) can be sufficient to fractionate the REE from the HFSE [e.g., Brenan et al., 1995; Stalder et al., 1998]. Available experimental D’s indicate that Zr (Hf ) is less efficiently retained in the slab than the HREE (Lu, inferred from Y and Yb), but not necessarily the MREE (e.g., Sm), despite the presence of residual rutile: DZr/Yb = 0.044, DZr/Sm = 1 [Stalder et al., 1998]; DZr/Y = 0.041, DZr/Sm = 0.2 [Ayers, 1998] (Stalder et al. [1998] assume 1.5% residual 11 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust rutile, Ayers [1998] assumes 1% residual rutile). In case of the study of Stalder et al. [1998], Zr (Hf ) is less efficiently retained in the oceanic crust than Yb (Lu), thus increasing the Lu/Hf in the oceanic crust, but leaving the Sm/Hf ratio unchanged. Using the Ds of Ayers [1998] both Y (Lu) and Sm do not fractionate from Zr (Hf ), despite DZr/Y and DZr/Sm 6¼ 0, because the absolute values of DSm, DY, and DZr are so high that, effectively, Sm, Y and Zr (Sm, Lu and Hf ) do not fractionate during sub-arc alteration for a large range of fluid/slab ratios. Contrary to that, empirical slab/fluid partition coefficients [McCulloch and Gamble, 1991] indicate that Zr (Hf ) is more effectively retained in the slab than Sm and Yb (Lu) so the Sm/Zr and Yb/ Zr (Sm/Hf, Lu/Hf ) ratios in the oceanic crust decrease during subduction. [26] Experimental studies of subduction zone processes indicate that 147Sm/144Nd ratios in the oceanic crust increase during subduction [Ayers, 1998; Kogiso et al., 1997; Stalder et al., 1998], in agreement with empirically determined Dslab/fluid [McCulloch and Gamble, 1991], but the magnitude of this increase is difficult to estimate. Dslab/fluid are rarely given for both Sm and Nd, but experimental and empirical studies indicate a higher mobility of the LREE compared to the MREE and HREE [Ayers, 1998; Kogiso et al., 1997; McCulloch and Gamble, 1991; Stalder et al., 1998]. The relative differences between D slab/fluid for LREE and MREE are large: DLa/DSm 0.035 [Stalder et al., 1998] and 0.01 [Ayers, 1998]. Absolute values of D’s are also variable and can change from values <1 to values >1 for La [Ayers, 1998; Stalder et al., 1998] and can be two orders of magnitude different for Sm. Kogiso et al. [1997] estimate that about 14% Sm and 31% Nd (56% La) are extracted from the oceanic crust. [27] Based on the above discussion, 147Sm/144Nd of the oceanic crust increase during sub-arc alteration, although there is significant uncertainty regarding the magnitude of that increase. Assuming that 147Sm/144Nd ratios between 0.19 and 0.22 are typical of the oceanic crust (see distribution in Figure 2a) and that 147Sm/144Nd further increase during subduction requires recycling ages to be >1.5 Ga (see Figure 2a and compare Tables 1 and 10.1029/2001GC000223 2). The presence of residual rutile is critical to LuHf fractionation during sub-arc alteration. Although HFSE (e.g., Hf ) are compatible in rutile, experimental slab-fluid partitioning studies suggest that Hf can be more or equally effectively extracted from the oceanic crust than either Lu or Sm, even in case of residual rutile (see above). Thus, 176 Lu/177Hf (and Sm/Hf ) ratios in the subducted slab are expected to increase in the oceanic crust during subduction. Most 176Lu/177Hf in the fresh oceanic crust are between 0.020 and 0.028 (Figure 2b), and are therefore too low to be suitable HIMU sources for recycling ages >1.5 Ga (see Figure 2b and compare Tables 1 and 2). Therefore, because Sm-Nd systematics suggest recycling ages >1.5Ga, subducted crust is likely to be an appropriate HIMU source only for the case where subduction alteration leads to increasing 176Lu/177Hf in the oceanic crust. Decreasing the 176Lu/177Hf ratios in the oceanic crust during subduction as suggested by empirical slab-fluid partition coefficient estimates [McCulloch and Gamble, 1991], on the other hand, would lead to very low 176Lu/177Hf ratios in the subducted crust which are only suitable for the HIMU source in case of recycling ages 1 Ga (Table 2). These young recycling ages, however, are difficult to reconcile with the recycling ages >1.5 Ga inferred from the Sm-Nd systematics (see above). 3.1.3.3. U-Th-Pb System [28] For the N-MORB, gabbro, and the bulk crust m, w, and k are too low compared to a 2 Ga old HIMU source (Figures 2d–2f ). For the altered crust, w and k are too low, while m is too high (Figures 2d–2f ). Thus for all four examples, w and k have to increase to become suitable HIMU sources, and except for the altered crust, m also has to increase. In contrast to other isotopic systems, this observation is almost independent of the assumed recycling age, as only some MORB with the highest w and k overlap those of the HIMU source (see distribution in Figures 2e and 2f; Table 2). Note that different source ages have little influence on the initial isotopic composition of the oceanic crust, because single or two stage source evolution models are very similar (see appendix B.2). 12 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust [29] Experimental slab-fluid partition coefficient ratios are between 51 and 81 for DU/DPb, and between 41 and 49 for DTh/DPb [Ayers, 1998; Brenan et al., 1994, 1995]. Kogiso et al. [1997] estimate that about 85% Pb, 29% U, and 38% Th are extracted from the oceanic crust. All experimental studies agree that Pb behaves more incompatibly than U and Th, thus increasing w and m in the oceanic crust, although the magnitude of this increase is highly variable [Ayers, 1998; Brenan et al., 1994, 1995; Kogiso et al., 1997]. [30] Partition coefficients estimated based on the composition of arc basalts suggest that U behaves more incompatible than Th and Pb during subduction (DU/DTh = 0.5, DU/DPb = 0.5; [McCulloch and Gamble, 1991]). Independent evidence that U behaves more incompatible than Th during subarc alteration is provided by the 238U excesses observed in arc lavas. The 238U excesses in arclavas are commonly attributed to metasomatism by a fluid derived from the subducted crust which is enriched in U relative to Th, i.e. Th is more efficiently retained in the subducted slab than U [e.g., Elliott et al., 1997; Gill and Williams, 1990, 1997; Turner et al., 1996]. Experimentally estimated slab-fluid partition coefficient ratios DU/DTh depend on the amount of garnet in the subducted slab (eclogite) and the oxygen fugacity during slabfluid interaction [Brenan et al., 1994, 1995]. DU/ DTh decreases with decreasing abundance of garnet in the subducted crust and increasing oxygen fugacity [Brenan et al., 1994, 1995]. DU/DTh is <1, i.e. U behaves more incompatible than Th, at high oxygen fugacities and modal proportions of garnet in the oceanic crust (=eclogite) <60% [Brenan et al., 1994, 1995]). The given DU/DTh of Ayers [1998] depends strongly on the amount of rutile (DUrutile = 89, DThrutile = 0.1) and can change from values >1 for modal abundances of rutile >0.5% to values <1 for modal abundances of rutile <0.5%. In the study of Kogiso et al. [1997], 29% U and 38% Th are extracted from the oceanic crust, suggesting that U is less incompatible than Th. Thus, although there is some disagreement on the relative partitioning of Th and U during sub-arc processes, the 238U excesses [Elliott et al., 1997; Gill and Williams, 1990, 1997; Turner et al., 1996] 10.1029/2001GC000223 observed in island arc lavas are considered to be strong evidence that U is more efficiently extracted from the oceanic crust during subduction than Th. [31] The k values in fresh oceanic crust (e.g., NMORB, gabbro and bulk crust) are lower than required for the HIMU source, almost independent of the assumed age of the HIMU source (Figure 2f ). That U behaves less incompatible during subarc alteration than Th is therefore also required for recycled oceanic crust to become suitable HIMU sources. The m and w in most basalts (Figures 2d and 2e) are also lower than required for the HIMU source, and the inferred increase of m and w during sub-arc alteration can transform subducted oceanic crust into suitable HIMU sources if the magnitude of increase of m and w (and k) is appropriate for the assumed recycling/source age. The large variability in existing estimates, however, prevents a more precise estimate of possible compositions and recycling ages. In the altered oceanic crust m is too high and w is too low to be an appropriate HIMU source. Assuming that little Th is extracted from the oceanic crust during subduction, w can only increase by preferential extraction of Pb compared to Th. This in turn leads to increasing m, and U has to be extracted almost quantitatively from the altered oceanic crust to yield appropriate m and w values. At the same time U extraction is limited by the k values, so that it is difficult to find U, Th and Pb mobility parameters that are in agreement with experimental studies (see above) and at the same time transform altered oceanic crust into suitable HIMU sources. 3.1.4. The Trace Element Composition of Recycled Igneous Oceanic Crust [32] Since the preferential removal of some elements during subduction (especially Pb, but also alkali elements and U) leads to distinct trace element signatures, another possible test for the plausibility of recycled oceanic crust as HIMU source comes from its trace element patterns. Ce/ Pb and Nb/U ratios in HIMU basalts reported by Chauvel et al. [1992, 1997] are higher than those in typical OIB [Newsom et al., 1986] and are at least qualitatively consistent with the required Pb and U loss during subduction. 13 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust 10.1029/2001GC000223 1000 calculated OIB melt PUM normalized 100 10 calculated OIB source 1 average Tubaii basalt average St. Helena basalt average Tristan da Cunha basalt average Society basalt 0.10 Cs Rb Ba Th U Nb Ta La Ce Pb Nd Sr Zr Hf Sm Eu Ti Gd Dy Y Er Yb Lu Figure 3. Shown is the trace element pattern of a calculated OIB source and its melt. The composition of the subducted crust is equal to those of the subduction-modified bulk oceanic crust (see section 3.1.2 and Tables 1 and 3), the degree of melting is 1% and assumes fractional melting of an eclogitic crust in the garnet-stability field (see spread sheet ‘‘TE_OIBmelts’’, and appendix B and auxiliary material for details of the calculations). The trace element pattern of the calculated OIB melt agrees fairly well with those of average HIMU basalts from Tubaii (compiled from the GEOROC database; http://georoc.mpch-mainz.gwdg.de) and St. Helena [Chaffey et al., 1989] (see also see spread sheet ‘‘TE_OIBmelts’’). Also shown are average EMI basalts from Tristan da Cunha (compiled from the GEOROC database; http://georoc.mpch-mainz.gwdg.de) and EMII basalts from Society [White and Duncan, 1995]. Normalizing values are the primitive mantle values (PUM) of McDonough and Sun [1995]. [33] As one example for a possible HIMU source derived from recycled oceanic crust, we have taken our estimate of the bulk oceanic crust composition (Tables 1 and 3) and assume a source and recycling age of 2Ga. The estimates of element mobility during sub-arc alteration are based on the mobility estimates of Kogiso et al. [1997], but are modified in order to achieve average present-day HIMU isotopic signatures (Table 3). The required changes in P/D ratios are large: 238U/204Pb, 232Th/204Pb, 232 Th/238U, 147Sm/144Nd, and 176Lu/177Hf have to increase by 26%, 228%, 160%, 11%, and 28%, respectively, and 87Rb/86Sr has to decrease by 68% (Table 3). [34] Melting of the subducted crust directly as an eclogitic assemblage produces trace element patterns similar to those of HIMU basalts for a degree of melting of about 1%, although with more pronounced Nb and Ta anomalies. In case the oceanic crust is assumed to be admixed to depleted or slightly enriched mantle (assuming less than 25% basalt in the mixture), the trace element patterns are also broadly similar to those of modern HIMU basalts (Figure 3), but even lower degrees of melting (<0.5%), depending on the amount of basalt in the mixture, are required (see also spread sheet ‘‘TE_OIBmelts.xls’’). Note that in the latter case, mixing of the subducted crust with ambient mantle will produce isotopic mixing trends between HIMU and the composition of the ambient mantle. For both cases, the trace element compositions of OIB require very low degrees of melting, even if produced entirely from enriched sources such as the subducted oceanic crust, which is in contrast to the larger degrees of melting inferred from major element systematics (several percent). 3.1.5. Recycling Igneous Oceanic Crust: Summary and Conclusions [35] It is virtually impossible to find a composition of the present-day oceanic crust that yields a HIMU-like isotopic composition without further 14 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust modification during sub-arc processing. This conclusion mainly stems from the Th/Pb systematics in the oceanic crust, which indicate that, almost independent of the assumed recycling age, the Th/Pb in most ridge basalts are too low to be comparable to those in the HIMU source. Thus, subduction plays a paramount role in understanding crust-mantle recycling and the composition of ancient recycled oceanic crust. [36] The Rb/Sr ratios decrease while the Sm/Nd (and likely the Lu/Hf ) ratios of the oceanic crust increase during subduction. All experimental studies agree that Pb is preferentially removed compared to U and Th, thus increasing the U/Pb and Th/Pb ratio. The U-Th fractionation is dependent on factors such as the abundance of garnet and rutile in the subducted slab, and the oxygen fugacity, but U-Th disequilibrium studies in island arc volcanics indicate that U is lost preferentially compared to Th during subduction (Th/U increases), which is required for recycled basalts to become suitable HIMU sources. [37] Although there has been substantial progress in quantifying element transfer during sub-arc processing of the oceanic crust, and experimental studies agree in most cases on the relative fractionation of elements, the magnitude of fractionation of parent-daughter elements pairs remains uncertain. Furthermore, there are few constraints on likely recycling ages and depending on the relative proportion of altered and fresh basalt and gabbro in the bulk subducted oceanic crust, the composition of the oceanic crust can be quite variable. Thus, it remains hard to make a general estimate of the present-day isotopic composition of ancient subduction-modified oceanic crust, as for all three relevant parameters (age, composition before and after subduction-processing) it remains plausible to assume a large range of values. [38] Some conclusions, however, are possible based on the analyses in section 3.1: (1) Hydrothermally altered basalt alone is unlikely to be a source of HIMU basalts, because Rb (U) loss during sub-arc processing is insufficient to transform hydrothermally altered crust into suitable 10.1029/2001GC000223 HIMU sources. (2) If the HIMU source mainly consists of subducted basalts, then very low degrees of melting (approximately <1%) are required based on the trace element systematics of HIMU-like basalts. (3) Subducted oceanic crust is only a likely HIMU source in case Lu/Hf ratios of the oceanic crust increase during subduction. (4) Ancient subducted crust can only be a HIMU source if U and Pb are removed preferentially to Th from the subducted slab. (5) There are likely to be large variations in the isotopic composition of ancient oceanic crust as a function of recycling age, composition and subduction modification (Figure 1). Thus, ancient subducted crust is likely to have a range of isotopic compositions. A narrow range of isotopic compositions (e.g., similar to HIMU: St. Helena and Austral plume) can only result if similar material is processed in a similar way at about the same time. Otherwise, oceanic crust with different initial composition and age would have to be processed differently as a function of time and composition, but always in such a way that the end-product has similar isotopic signatures. 3.2. Recycling of Oceanic Crust Plus Sediment 3.2.1. Modeling the Isotopic Composition of Recycled Crust Plus Sediment [39] In order to model the effects of sediment subduction, 2 and 10% of subduction-modified sediment with different compositions (we use the 28 bulk sediment compositions of Plank and Langmuir [Plank and Langmuir, 1998]) are mixed with subducted oceanic crust). We use the subducted bulk igneous crust derived in the previous section (a mix of altered and fresh basalts and gabbro), which has HIMU isotopic signatures (Table 3). Recycling and source ages of 2 Ga are assumed for both the basalt and the sediment (a single stage evolution for the sediment reservoir is assumed in order to model the initial isotopic composition of the sediment; see appendix and spread sheet ‘‘basalt + sediment_recycling’’) (auxiliary material). To estimate the composition of subducted sediments, mobility estimates for sediment-melting at 900C are used (Table 3), 15 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust which are calculated according to the parameters given by Johnson and Plank (their Tables 1 and 8, formula for mobility as given in paragraph 38), since there is some geochemical evidence that sediments melt during subduction [e.g., Elliott et al., 1997; Johnson and Plank, 2000]. [40] Sediment melt-interaction during subduction changes the Sm/Nd and Th/U ratios by less than 1% [Johnson and Plank, 2000]; U/Pb and Th/Pb decrease by about 20% relative to unmodified sediments, whereas the Rb/Sr and Lu/Hf ratios increase by about 21% and 47%, respectively (Table 2). Sediment-fluid exchange [Johnson and Plank, 2000] results in an increase of Th/U, U/Pb, Th/Pb and Sm/Nd by about 3%, 9%, 11%, and 6%, whereas Rb/Sr and Lu/Hf decrease by 3% and 6% compared to unmodified sediments, respectively (Table 2). Thus, the changes caused by sedimentmelt or sediment-fluid interaction lead to relatively minor differences in the isotopic composition compared to unmodified sediments (at least for recycling ages of 2Ga, see below). In contrast, required changes to transform the bulk igneous crust into a suitable HIMU source are substantially larger (see above). [41] Rather than mixing basalts and sediment, most previous models [e.g., Chauvel et al., 1992; Hart and Staudigel, 1989; Rehkämper and Hofmann, 1997] calculate the isotopic composition of the sediment end-member only, implying that the composition of the bulk recycled crust (sediment and basalt) is dominated by the sediment. While this is true for the most incompatible elements, which have significantly higher concentrations in sediments compared to basalts (Cs, Rb, K, U, Th, Pb,. . .), especially the middle and heavy REE (SmLu) and some HFSE (e.g., Zr, Hf ) have similar concentrations in sediments and basalts (Figure 5). As a consequence, mixtures of basalt and sediment can differ significantly in composition and develop different isotopic compositions compared to the pure sediment (see spread sheet ‘‘basalt+sediment_recycling’’ which gives both the basalt and sediment end-member and a range of mixtures between the basalt and sediment). Results of the modeling of basalt and sediment are shown in Figure 4 (see figure caption, appendix B and auxiliary material, 10.1029/2001GC000223 and spread sheet ‘‘basalt + sed_recyling’’ for details of the modeling). 3.2.2. Effect of Sediment Addition [42] Compared to recycled igneous crust alone (section 3.1), the effect of sediment addition is largest for Pb and Sr isotopes, and smallest for Nd and Hf isotopes. Note that the difference in concentration between average MORB [Hofmann, 1988] and average subducted sediment (GLOSS [Plank and Langmuir, 1998]) decreases in the order Pb > Sr > Nd > Hf: Pbsed/Pbbasalt 40; Srsed/Srbasalt 2.9; Ndsed/Ndbasalt 2.4; Hfsed/Hfbasalt 1.4 (Figure 5). Compared to MORB, sediments have similar Lu/Hf and Th/Pb ratios; Sm/Nd and U/Pb ratios are lower, and Rb/Sr and Th/U ratios are higher than in the basalts (Figure 6). Sediment-melt interaction during subduction leaves the Th/U and Sm/Nd ratios relatively unchanged, but increases the Rb/Sr and Lu/Hf ratios and decreases the U/Pb and Th/Pb ratios. As a consequence, the differences in P/D ratios and resulting isotopic differences in ancient recycled sediments and basalts are enhanced by sub-arc alteration. [43] Because of the low U/Pb ratio and the high U and Pb concentrations in sediments compared to basalt, the Pb isotopic composition of the bulk subducted crust decreases dramatically as a function of the amount and composition of the added sediment. As for MORB, U/Pb ratios in the sediments correlate positively with the Th/Pb ratios, but neither U/Pb nor Th/Pb correlate with the Th/U ratios. Since U/Pb and Th/Pb ratios are decreased by similar amounts during sediment-melt interaction, low 206Pb/204Pb ratios in most ancient recycled sediments are associated with low 208Pb/204Pb ratios. The low values of Th/Pb in the sediments (values comparable to MORB) and the high Pb contents therefore lead to 206,208Pb/204Pb ratios in subducted sediments that are, in general, lower than those in OIB, even if the sediment is mixed with basalts with HIMU-like compositions as for the examples in Figure 4 and even for small amounts of sediment (<5 –10% sediment). Note that the estimates of Pb mobility during subduction of Johnson and Plank [2000] are subject to technical difficulties, so that the changes in U/Pb and Th/Pb 16 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 0.5134 MORB a 0.5132 143Nd/144Nd 0.5130 EMII 0.5128 HIMU 0.5126 0.5124 GLOSS EMI 0.5122 average SAS 0.5120 0.702 0.704 0.706 0.708 0.710 0.712 0.714 87Sr/86Sr 42 208 Pb/204 Pb 41 b HIMU EMII 40 10% sediment REE rich sediments SiO2 rich sediments CaO rich sediments other sediments EMI 39 38 2% sediment REE rich sediments SiO2 rich sediments CaO rich sediments other sediments 37 MORB 36 subduction-modified MORB 35 15 16 17 18 19 20 21 22 23 24 206 Pb/204 Pb Figure 4. Various isotope ratio diagrams show the effects of sediment recycling on the present-day Sr, Nd, Hf, and Pb isotopic composition of ancient bulk subducted crust. 2 and 10% of subduction-modified sediment [Johnson and Plank, 2000; Plank and Langmuir, 1998] are mixed with the bulk igneous crust (see section 3.1.2 and Tables 1 and 3). Calculations assume a recycling age of 2Ga. Also shown are the mixing lines between the bulk igneous crust and average slowly accumulating sediment (SAS = ‘‘pelagic’’ sediment, see spread sheet ‘‘basalt + sediment_recycling’’) and global subducted sediment (GLOSS [Plank and Langmuir, 1998]), respectively. Tick marks are for 2 and 10% sediment. For further details of the calculation see section 3.2.1., spread sheet ‘‘basalt + sediment_recycling’’, and appendix B and auxiliary material. See section 3.2 for a detailed discussion. Plotted MORB and OIB data and references as in Figure 1. ratios during subduction might be different [see Johnson and Plank, 2000 for details]. However, 206 Pb/204Pb-208Pb/204Pb systematics in OIB would require the subducted sediments to have higher U/ Pb and Th/Pb but lower Th/U ratios. This is only possible if the Pb mobility is increased and at the same time the U but not the Th mobility decreased, which is considered unlikely. 17 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 0.5134 143Nd/144Nd c MORB 0.5132 0.5130 0.5128 HIMU 0.5126 EMII 0.5124 EMI 0.5122 0.5120 15 16 17 18 19 20 21 22 23 24 206Pb/204Pb 0.714 d 0.712 87Sr/86Sr 0.710 0.708 EMII EMI 0.706 HIMU 0.704 MORB 0.702 15 16 17 18 19 20 21 22 23 24 206 Pb/ 204 Pb Figure 4. (continued) [44] The higher Rb/Sr and lower Sm/Nd ratios in sediments compared to basalts lead to very high 87 Sr/86Sr and low 143Nd/144Nd ratios in ancient subducted sediments. In addition, the Sr (and to a lesser degree the Nd) concentrations in sediments are substantially higher than those in basalts (see above), so that the combined Sr and Nd isotope systematics in the ancient recycled crust are very sensitive to the amount of sediment. Note that calculated 87Sr/86Sr in the bulk subducted crust are only within the range of OIB values for small amounts of sediment (<5%; Figures 4a and 4d). [45] Calculated 143Nd/144Nd ratios in the bulk subducted crust fall well within the range of those in OIB, and in particular, EMI and EMII type basalts. However, combined Nd-Sr and Nd-Pb 18 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 0.2836 MORB 0.2834 177Hf/176Hf 0.2832 EMII 0.2830 HIMU 0.2828 0.2826 EMI e 0.2824 0.2822 0.5120 0.5122 0.5124 0.5126 0.5128 0.5130 0.5132 0.5134 143 Nd/ 144 Nd Figure 4. (continued) isotope systematics (Figures 4a, 4c, and 4e) are rarely appropriate for OIB, which is an effect of the low Pb and high Sr isotope values of the subducted sediment. 176Hf/177Hf ratios in the bulk subducted crust for all but the special cases of sediments with high Lu/Hf ratios deviate little from those in the pure oceanic crust (see below, Figure 4e) and are by itself not a very sensitive tracer of subducted sediments. Combined Nd and Hf isotope systematics show that in case less than about 10–15% subducted sediments are mixed with a basalt that will develop HIMU signatures, resulting Nd and Hf isotope ratios tend to plot within or slightly below the ‘‘mantle array’’ in a Nd-Hf isotope diagram (Figure 4e). Nd and Hf isotope compositions that plot above the ‘‘mantle array’’ only result in case large amounts of sediment (>10–15%) and/or sediments with high Lu/ Hf ratios are mixed with the oceanic crust (see below, Figure 4e). 3.2.3. Differences in Isotopic Composition Due to Different Sediments? [ 46 ] The compositions of EMI and EMII-type basalts have often been explained by recycling of sediments. Both EMI and EMII-type basalts are characterized by high 87Sr/86Sr and low 143Nd/144Nd and 176 Hf/ 177 Hf ratios; the 143 Nd/ 144 Nd and 176 Hf/177Hf ratios are systematically lower in EMI compared to EMII-type basalts. The most significant isotopic difference between EMI and EMII-type basalts are their Pb isotope systematics: EMI-type basalts have variable 206Pb/204Pb, and high and almost constant 208 Pb/ 204 Pb ratios; EMII-type basalts have variable 208Pb/204Pb for relatively constant 206Pb/204Pb ratios with intermediate values (see Figures 1 and 4). The time integrated Th/U ratios in both EMI and EMII-type basalts (as expressed by (208Pb/206Pb)* = (208Pb/204Pbmeasured 29.476)/(206Pb/204Pbmeasured 9.307); [Galer and O’Nions, 1985]) are variable, but are higher in EMI than in EMII. [47] These isotopic differences between EMI and EMII-type basalts have often been attributed to different sediment compositions; ‘‘pelagic’’ sediments have been favored for the EMI source and ‘‘terrigenous’’ sediments for the EMII source (see references given in the introduction). While the classification into pelagic and terrigeneous sediments is a useful genetic classification, sediments in either category display a large range of chemical compositions relative to the total compositional variability observed in marine sediments, so that this classification is less useful for geochemical 19 of 33 3 Geochemistry Geophysics Geosystems G stracke et al.: recycling oceanic crust 10.1029/2001GC000223 1000 subducted sediments PUM-normalized 100 10 1 N-MORB, Hofmann, 1988 GLOSS REE-rich sediments CaO-rich sediments SiO2-rich sediments 0.1 Cs Rb Ba Th U Nb Ta La Ce Pb Nd Sr Zr Hf SmEu Ti Gd Dy Y Er Yb Lu Figure 5. Plot of the trace element patterns of bulk subducted sediments [Plank and Langmuir, 1998]. Most trace elements in bulk subducted sediments show about a ten-fold variation (gray area), with decreasing variability with increasing compatibility (compatibility increases from left to right). Subducted sediments dominated by slowly accumulating sediments (e.g., red clays with a large proportion of metalliferous components or phosphate) are REE enriched. Sediments with a large proportion of biogenic siliceous or carbonate components are characterized by depletions in HSFE (e.g., Hf, Zr, Nb, Ta, Th, Ti), and carbonate dominated sediments show characteristic Sr and Ba enrichments and Rb and Cs depletions. Shown for comparison is the average N-MORB of Hofmann [1988]. Normalizing values are the primitive mantle values of McDonough and Sun [1995]. purposes [see Plank and Langmuir, 1998]. In general, the geochemical composition of marine sediments is determined by three factors: the abundance of biogenic and detrital phases, the nature of the source of the detrital components, and the sedimentation rate (see Plank and Langmuir [1998] for a detailed discussion). Systematic differences in the isotopic composition of the bulk recycled crust as a function of sediment type, as suggested to explain the differences between the EMI and EMII sources, are only expected in case the P/D ratios in sediments (e.g., Rb/Sr, Sm/Nd, U/ Pb. . .) vary systematically as a function of sediment type. [48] Subducted sediments dominated by slowly accumulating sediment (e.g., red clays with a large proportion of metalliferous components or phosphate; referred to as ‘‘SAS’’ = Slowly Accumulating Sediments in the following) scavenge REE from seawater and are enriched in REE compared to most sediments [e.g., Ben Othman et al., 1989; Plank and Langmuir, 1998] (Figure 5). They are, therefore, characterized by distinct trace element ratios between REE and other trace elements, such as high La/Nb, or (Lu, Sm)/Hf ratios (Figures 5 and 7). SAS also tend to have some of the lowest U/Pb and Th/Pb ratios for the bulk sediment compositions considered here. Carbonate dominated biogenic sediments show characteristic Sr and Ba enrichments and Rb and Cs depletions leading to high Sr/Nd and Ba/Th ratios, and the lowest Rb/Sr ratios of all sediments (Figures 5 and 7). Silica dominated biogenic sediments are depleted in Sr (Figure 5). Both sediments dominated by biogenic siliceous or carbonate components are also depleted in HFSE relative to most sediments due to the low concentrations of HFSE in seawater and the incompatibility of HFSE in the mineralogic structure of marine organisms. Thus, both SAS and most, but not all, biogenic sediments have distinctively higher Lu/Hf ratios compared to 20 of 33 3 Geochemistry Geophysics Geosystems G a 160 30 20 10 120 80 40 0 0 0.10 0.14 0.18 0.22 0.26 Frequency sediment/MORB MORB Frequency MORB MORB d 30 sediment 20 10 0 0 0.30 4 8 12 16 20 24 28 32 36 40 44 48 52 1 4 7 S m / 1 4 4 Nd 2 3 8 U / 2 0 4 Pb 40 b 30 MORB 20 0.1-0.47 10 200 20 100 0 0.04 sediment 10 0 0.02 0.06 0.08 0 0.1 0 10 20 30 40 50 1 7 6 L u / 1 7 7 Hf 60 70 80 90 100 2 3 2 T h / 2 0 4 Pb 20 20 120 MORB c Frequency sediment range in MORB 16 Frequency sediment e 30 sediment 0 MORB Frequency sediment/MORB 300 Frequency MORB Frequency sediment 40 12 sediment 8 4 0 f 16 12 sediment 8 Frequency MORB Frequency sediment 40 200 40 sediment 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 0.6 1.0 1.4 1.8 2.2 2.6 3.0 3.4 87Rb/86Sr 40 4 0 0 0 0.2 80 0 2 4 6 8 10 12 14 16 232Th/238U Figure 6. Histograms showing the variation and frequency of parent daughter (P/D) ratios in present-day MORB glasses as compiled from the LDEO petrological database http://petdb.ldeo.columbia.edu/petdb) compared to the range of P/D ratios in modern subducted sediments [Ben Othman et al., 1989; Plank and Langmuir, 1998]. Sm/Nd and U/Pb ratios in modern sediments are lower, whereas Rb/Sr ratios are substantially higher than those in MORB. Lu/Hf and Th/Pb ratios in MORB and sediments show a similar range and Th/U ratios in the sediment tend to be higher and are more variable than those in MORB. other sediments (Figures 5–7), and, upon aging, will develop high 176Hf/177Hf ratios. The typically high Lu/Hf ratios of SAS and most biogenic sediments, however, are the only distinct difference in P/D ratios as a function of sediment type (Figure 7), and most of the bulk sediment compositions considered here [Plank and Langmuir, 1998] show no systematic variation of P/D ratios with sediment type (Figures 6 and 7). [49] SAS have often been classified as ‘‘pelagic’’ sediments in previous studies [e.g., Ben Othman et al., 1989; Chauvel et al., 1992; Rehkämper and Hofmann, 1997; Weaver, 1991]. While the Sr-Nd 21 of 33 3 Geochemistry Geophysics Geosystems 176Lu/177Hf G stracke et al.: recycling oceanic crust 0.125 0.125 0.100 0.100 0.075 0.075 0.050 0.050 0.025 0.025 0.000 0.000 0.1 0 0.11 0.12 0.13 0.14 0.15 0.16 0.17 0.125 0.125 0.100 0.100 0.075 0.075 0.050 0.050 0.025 0.025 0.000 0 4 8 10 20 30 40 50 232Th/204Pb 147Sm/144Nd 176Lu/177Hf 10.1029/2001GC000223 12 16 0.000 0.01 0.1 1 10 87Rb/86Sr 238U/204Pb Plank and Langmuir, 1998 REE rich sediments SiO2 rich sediments CaO rich sediments other sediments Ben Othman et al., 1989 Figure 7. Plot of parent-daughter (P/D) ratios in modern subducted sediments [Ben Othman et al., 1989; Plank and Langmuir, 1998]. Some of the bulk subducted sediments [Plank and Langmuir, 1998] have high Lu/Hf ratios due either to REE enrichment in slowly accumulating sediments or HFSE depletion in CaO and SiO2 rich sediments, but have otherwise similar P/D ratios. isotope evolution of ‘‘average’’ SAS roughly follows the trend of EMI basalts (Figure 4a), they develop the most radiogenic Hf isotope ratios of all sediments (Figure 4e). The Hf-Nd isotope characteristics of SAS are only similar to those of EMI and EMII-type basalts for small amounts of sediment (<10%). SAS also have the most unradiogenic Pb isotope ratios of all sediments, for both 206 Pb/204Pb and 208Pb/204Pb (Figures 4b, 4c, and 4d). The most characteristic feature of EMI-type basalts, the high and constant 208Pb/204Pb ratios for given 206Pb/204Pb, are therefore not a typical feature of ancient ‘‘pelagic’’ sediments (see Figures 4b and 5–7; see also [Ben Othman et al., 1989; Plank and Langmuir, 1998]). Moreover, of all sediments, SAS are not only the presently least abundant but are generally also of very small volume [Plank and Langmuir, 1998], so that recycling large amounts of such sediments over geologic time seems unlikely. 22 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust 10.1029/2001GC000223 1000 calculated OIB melt 10 calculated OIB source: igneous crust + 5%sediment PUM normalized 100 1 calculated igneous crust + 2%GLOSS calculated igneous crust only average Tristan da Cunha basalt average Society basalt 0.1 Cs Rb Ba Th U Nb Ta La Ce Pb Nd Sr Zr Hf Sm Eu Ti Gd Dy Y Er Yb Lu Figure 8. Shown are the trace element patterns of calculated OIB sources, which are derived from melting of recycled oceanic crust plus various amounts of average subduction-modified sediment (0, 2, and 5%; sediment = GLOSS [Johnson and Plank, 2000; Plank and Langmuir, 1998]). Also shown is the melt of the OIB source consisting of the bulk igneous crust (see section 3.1.2 and Tables 1 and 3) and 5% of sediment. Calculations assume a degree of melting of 4%, fractional melting of an eclogitic assemblage in the garnet-stability field. See Table 3 for basalt and sediment compositions and for mobility estimates. See spread sheet ‘‘TE_OIBmelts’’, and appendix B and auxiliary material for details of the calculations. With the exception of Pb, the trace element pattern of the calculated melt agrees fairly well with those of average EMI basalts from Tristan da Cunha (compiled from the GEOROC database; http://georoc.mpch-mainz.gwdg.de) and EMII basalts from Society [White and Duncan, 1995] (see also spread sheet ‘‘TE_OIBmelts’’). Normalizing values are the primitive mantle values of McDonough and Sun [1995]. [50] As an analog for ‘‘terrigeneous’’ sediments [Ben Othman et al., 1989], we take the average composition of global subducted sediments (GLOSS [Plank and Langmuir, 1998]). GLOSS and the other non-SAS sediments form a trend that is roughly parallel to those of EMII-type basalts in a Nd-Sr isotope diagram (Figure 4a), but for the Sr isotope ratios to be similar to those of EMII-type basalts, the amount of sediment in the bulk subducted crust needs to be smaller than about 10%. GLOSS does not develop the radiogenic Hf isotope composition of SAS. The Nd-Hf isotope compositions of GLOSS and the other non-SAS sediments form a trend away from HIMU toward the ‘‘mantle array’’. Sediment proportions >10% are needed to develop compositions with higher 176Hf/177Hf ratios for given 143Nd/144Nd ratios than those of the ‘‘mantle array’’. The 206Pb/204Pb and 208Pb/ 204 Pb ratios in non-SAS type sediments vary widely, but are generally lower than in most OIB. Sediments with a small range in U/Pb but variable Th/Pb ratios would be required to reproduce the variable 208Pb/204Pb for almost constant 206Pb/ 204 Pb ratios that are typical of EMII-type basalts, but no type of sediment with these characteristics can presently be identified. 3.2.4. Trace Element Composition of Subducted Oceanic Crust Plus Sediment [51] The distinct trace element concentrations of subducted sediments relative to those of fresh or subduction-modified basalts have a considerable leverage on the trace element composition of the bulk subducted crust (see spread sheet ‘‘TE_OIBmelts’’). Figure 8 shows the effect of addition of 2, 5, and 10% of sediment (GLOSS [Plank and Langmuir, 1998]) to the igneous oceanic crust (the bulk igneous crust as calculated in section 3.1, see Table 3). The effect of sediment addition is largest for Pb; only about 3% of sediment erase the pronounced negative Pb anomaly of the subducted igneous crust. Cs, Rb, Ba, Th, U, and La, and Ce 23 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust are also strongly affected by the addition of small amounts of sediment (<10%), whereas Nb, Ta, the MREE and HREE, and Sr, Zr, and Hf are hardly affected. Therefore, sediment addition to the igneous crust makes the trace element pattern steeper, causes positive Pb anomalies, and for >5% sediment, Nb and Ta become depleted relative to neighboring elements. [52] Melting of the subducted crust with minor amounts of sediment (<5%) directly as an eclogitic assemblage produces trace element patterns similar to those of EM-type basalts for degrees of melting <5%, although with positive Pb anomalies and more depleted HREE. In case the bulk subducted crust is assumed to be admixed to depleted or slightly enriched mantle, the trace element patterns can also be similar to those of modern EM-type basalts. In this case, different combinations of the amount of sediment in the bulk subducted crust and the amount of bulk subducted crust in the mantle source (<25%) are possible, but lower degrees of melting (<1%) are required compared to melting of the subducted crust as an eclogitic assemblage, depending on the amount of bulk subducted crust in the mixture (see also spread sheet ‘‘basalt + sediment_recycling’’). As in case of directly melting the bulk subducted crust, however, positive Pb anomalies and depletions in Nb and Ta result. Note that mixing of the bulk subducted crust with ambient mantle will produce isotopic mixing trends between and the composition of the bulk subducted crust and those of the ambient mantle. [53] The trace element patterns of average HIMU and EM-type basalts are very similar (Figure 3, see spread sheet ‘‘TE_OIBmelts’’). Sediment addition to the igneous subdcuted crust produces steeper trace element patterns than for the igneous crust alone. Therefore, progressively larger degrees of melting are required with increasing amount of sediment to account for the similar slopes of the trace element patterns of EM-and HIMU-type basalts, in case the isotopic difference between HIMU and EM-type basalts is assumed to be the addition of sediment. However, even for only a few percent of sediment in the bulk subducted crust (>3%), positive Pb anomalies result, which is 10.1029/2001GC000223 contrary to the negative Pb anomalies observed in EM-type basalts. 3.2.5. Recycling of Oceanic Crust Plus Sediment: Summary and Conclusions [54] The initial compositional difference between sediments and the igneous oceanic crust (Figure 6) is enhanced by subduction modification. The subduction of minor amounts of sediment (<10%) in addition to the igneous oceanic crust leads to lower Nd and Pb and higher Sr isotope ratios, whereas Hf isotope ratios are, in general, relatively little changed compared to recycled basalt alone (Figure 4). Of all the isotopic systems investigated, the combined Sr and Nd isotope systematics of the bulk subducted crust (igneous crust + sediments) are most compatible with the isotopic composition of EM-type basalts. The Nd and Sr isotope composition of average SAS (often labeled ‘‘pelagic’’ sediment) seems to follow the trend of EMI-type basalts, whereas other sediment compositions (including GLOSS [Plank and Langmuir, 1998], often labeled ‘‘terrigeneous’’sediments) are more compatible with the trend of EMII-type basalts. The trends of EM-type basalts in Sr-Pb and Nd-Pb isotope space could also be interpreted as being part of a mixing hyperbola between a HIMU source (consisting of recycled basalt) and sediment. The Pb isotope values, however, are too unradiogenic to be compatible with this interpretation. As for subduction of the igneous oceanic crust alone, the Pb isotope systematics are therefore most difficult to reconcile with the notion of ancient recycled crust in OIB sources. Even if mixed with a HIMU source as for the examples in Figure 4, the high Pb contents and low U-Th/Pb ratios in sediments lead to very unradiogenic Pb isotope ratios, even for small amounts of sediment (<5%). Both sediment-melt and sediment-fluid alteration appear to affect the Th/Pb and U/Pb to a similar degree, so that the U/Pb and Th/Pb characteristics of the sediments are preserved during subduction processing. Therefore, the required P/D characteristics of EMI and EMII-type basalts (variable U/Pb but relatively constant Th/Pb for EMI and variable Th/Pb but relatively constant U/Pb for EMII-type basalts) would have to be an inherent characteristic of specific types of sediment, but no such group of 24 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust sediments can presently be identified. As a consequence, the characteristic Pb isotope features of EMI and EMII basalts are difficult to reconcile with ancient recycled sediment in their source. [55] The trace element pattern of the bulk subducted crust (igneous crust + sediment) are sensitive to the amount of added sediment. The addition of sediment to the igneous crust results in low Nb and Ta and high Pb concentrations relative to neighboring elements, and, in general, in a progressively steeper trace element pattern with increasing amount of sediment (Figure 8). Increasing amounts of sediment in the source therefore require an increasing degree of melting (F) to maintain a constant slope of the trace element pattern of the associated melt. EM and HIMU-type basalts have trace element patterns with very similar slopes (Figure 3; see also spread sheet ‘‘TE_OIBmelts’’). Thus, if the difference between HIMU and EM-type basalts is assumed to be a larger amount of sediment in the EM sources, higher F are required for EM-type basalts to compensate for the steeper trace element pattern of their sources. Because most OIB underwent substantial amounts of fractional crystallization, it is difficult, however, to find independent support for this notion in the major element chemistry of EM and HIMU-type basalts. [56] As for the subducted igneous crust alone, the ancient subducted crust including sediment is expected to show large variability as a function of composition, age, and differences in subduction modification. Thus, as in case of the HIMU signature, creating similar isotopic signatures by recycling oceanic crust plus sediment requires that very specific sediment compositions have to be recycled at similar times in order to yield similar isotopic signatures upon aging. 4. Conclusions [57] Creating HIMU sources by recycling subduction-modified oceanic crust requires substantial modification of the trace element budget of the oceanic crust during subduction. The required modifications are qualitatively consistent with dehydration processes in subduction zones, but a better quantitative understanding of subduction 10.1029/2001GC000223 zone processes is needed for a more precise estimate of the final composition of the subducted igneous crust. Although the addition of sediment tends to produce signatures which broadly point toward EM-type OIB for the Sr and Nd isotope system, suggesting that the sources of EM-type basalts may contain a component with crustal affinities, this notion is difficult to reconcile with the Pb isotope systematics. [58] No matter if sediment is involved or not, recycling of oceanic crust results in a large isotopic variability as a function of composition, recycling age and possible differences in subduction alteration. As a consequence, if the isotopic signatures of OIB are attributed to recycling of oceanic crust (with or without sediment), each OIB source is likely to represent a unique combination of composition, age and subduction modification of the ancient recycled crust. Even for this scenario, however, the grouping of OIB into several ‘‘families’’ or groups with similar isotopic composition [e.g., White, 1985; Zindler and Hart, 1986] suggests that crustal recycling cannot be an entirely random process, but that there must either be some broadly reproducible combination of composition, age and alteration, or that there is some as of yet unknown physical and/or chemical process that aids in reducing the apparent variability of the subducted crust. [59] Attributing mantle heterogeneity to a few endmembers (e.g., HIMU, EMI and EMII), which ultimately originate from crustal recycling, on the other hand, requires a very specific combination of age and composition for each end-member. Therefore, one single event for each proposed endmember, rather than continuous subduction and recycling of oceanic crust into the Earth’s mantle would have to be invoked to attribute mantle heterogeneity to a few end-members in the context of crustal recycling. These constraints, however, can be somewhat relaxed when taking into account that the isotopic composition of the proposed endmembers might range to more extreme values than those observed in OIB. In this case, a larger number of different recycling ages and/or compositions may be allowed. Alternatively, the alteration, sedimentation, and subduction processing of 25 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust oceanic crust could be a by far better defined process than observation would seem to indicate, resulting in surprising uniformity among discrete parcels of recycled material, or, the intramantle disaggregation and mixing of compositionally diverse recycled materials has an averaging affect and leads to the small number of relatively welldefined enriched compositions evidenced in MORB and OIB isotopic trajectories. In this case, physical and chemical processes have to be identified which are responsible for creating the different end-member compositions. [60] It should be pointed out that based on the assumption that crustal recycling is the major processes to create mantle heterogeneity, each view, those of only a few or those of many different isotopic components in the mantle, appears to require that the mantle operates in a very different manner. Assuming only a few end-members requires that the original variability of the oceanic crust is averaged whereas the creation of many different components requires the ability to preserve the heterogeneity of the subducted crust on some level over millions to billions of years. Either view has to be compatible with geophysical constraints on the preservation of chemical heterogeneities in the mantle and only a combination of geochemical and geophysical constraints can be successful in developing a more focused picture of mantle heterogeneity and mantle dynamics. Appendix A: Os Isotopes as a Tracer of Crustal Recycling [61] A detailed quantitative analytical treatment of crustal recycling for Os isotopes is not presented. This is because owing to the relatively limited amount of available data and the challenging analytical task, the Os isotope composition (and evolution) of the mid ocean ridge basalt (MORB) and Bulk Earth (BE) reservoir is not as well constrained as for other isotopic systems (Sr, Nd, and Pb isotopes), and a quantitative treatment is even more problematic (see below; see Hauri and Hart [1993] and Roy-Barman and Allègre [1995] for quantitative modeling). 10.1029/2001GC000223 [62] 187Os/188Os ratios in OIB higher than those in BE have often been regarded as a unique tracer for recycled material in the Earth’s mantle because to date, Re/Os ratios higher than those in BE have only been observed in crustal rocks (oceanic and continental [e.g., Hauri and Hart, 1993; Hauri et al., 1996; Lassiter and Hauri, 1998; Reisberg et al., 1993; Roy-Barman and Allègre, 1995; Widom et al., 1999; Widom and Shirey, 1996]). However, recent data for continental and oceanic rocks [Peucker-Ehrenbrink, 2001] show that it is difficult to distinguish between ancient oceanic crust and continentally derived material based on Os isotope ratios, and while Os isotope ratios may be a good indicator of recycled material in general, they do not appear to be discriminative between different types of recycled materials (oceanic crust and sediment). Also, extremely high Re/Os ratios ( 200) recently reported in oceanic gabbros lead to very radiogenic 187Os/188Os ratios (187Os/188Os = 1) over geologically short time (50m.y.) [Hart et al., 1999] suggesting that such a component cannot be present in pristine form in any OIB source. [63] Furthermore, recent Os isotope studies in OIB have cast some doubt on the uniqueness of attributing radiogenic 187Os/188Os ratios to recycled crust. It has been suggested that the radiogenic 187 Os/188Os ratios in OIB can also be a signature of core-mantle interaction [e.g., Bennett et al., 1996; Brandon et al., 1998, 1999; Walker et al., 1995; Widom and Shirey, 1996]. An ‘‘enriched plume component’’ with radiogenic Os isotope values (187Os/188Os = 0.130 0.135) common to many OIB and equivalent to the lower mantle has also been invoked [Shirey and Walker, 1998; Widom and Shirey, 1996]. As an alternative to both the crustal recycling and core-mantle interaction models, melting of variable amounts and/or compositions of residual sulfide have recently been suggested as an explanation for the Os isotope and Re and platinum group element (PGE) systematics in Hawaiian basalts [Bennett et al., 2000]. [64] Therefore, although Os isotope systematics are a potentially powerful tracer of ancient recycled crust, alternative explanations have to be considered and it even appears possible that radiogenic 26 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust 187 Os/188Os values in OIB may not be a unique feature of recycled crust. Table A.1. MORB Source Evolution: A Comparison of Different Approachesa Age (Ga) Appendix B: Quantitative Modeling of the Composition of Ancient Recycled Oceanic Crust [65] In order to determine the present-day isotopic composition of ancient recycled crust, the age and the initial isotopic composition, as well as the parent-daughter ratio (P/D) for each isotopic system investigated have to be known. The following is a brief description of how each of these variables is determined using the most recent constraints on the processes involved and a comparison with the approach taken in previous studies. The provided spread sheets give the reader access to all the calculations and input parameters. The reader is encouraged to change the modeling parameters according their own preference and to investigate the influence on the final composition of the recycled crust. For each spread sheet, a ‘‘user guide’’ is provided in auxiliary material which explains its general outline. Remaining questions about the modeling and the use of the spread sheets should be addressed to the first author. B.1. Age of the Recycled Crust [66] The age of the recycled crust plays a crucial role, as it is one of the main factors that determine its initial isotopic composition. Unfortunately, due to the lack of more detailed constraints on mantle dynamics, the age of the recycled oceanic crust is essentially a free parameter. However, as shown by Hauri and Hart [1993], the Pb isotopic composition of MORB and HIMU basalts indicate that recycling ages are likely to be between 0.77 and 2.1Ga. B.2. Initial Isotopic Composition of the Recycled Crust B.2.1. Oceanic Crust [67] For each isotopic system, the initial isotopic composition of the recycled basalts is assumed to be equal to those of the MORB source at the time of recycling. The Pb isotope evolution of the This study Hart and Chauvel Rehkämper Hauri and Staudigel et al. and Hofmann Hart 87 0 1 2 3 4 4.5 0.7027 0.7026 0.7024 0.7022 0.7016 0.7010 0.7004 0.6997 Sr/86Sr 0.7026 0.7018 0.7010 0.7002 0.6994 0.6990 0.7022 0.7023 0.7024 0.5132 0.5117 0.5102 0.5087 0.5072 0.5067 0.5133 0.5117 0.5100 143 0 0.5132 1 0.5116 2 0.5100 3 4 4.55 0.5133 0.5118 0.5104 0.5089 0.5074 Nd/144Nd 0.5132 0.5117 0.5102 0.5087 0.5072 0.5067 0.7025 0.7017 0.7010 0.7002 0.6994 0.6990 176 0 1 2 0.28335 0.28243 0.28148 0 1 2 3 4 4.55 17.72 16.34 14.73 12.86 10.66 9.31 17.30 16.00 14.48 12.71 10.64 0 1 2 3 4 4.55 37.34 35.76 34.10 32.36 30.52 29.48 37.19 35.63 33.99 32.26 30.45 Hf/177Hf 0.28355 0.28253 0.28148 206 Pb/204Pb 17.61 16.25 14.67 12.81 10.65 9.31 Pb/204Pb 37.25 35.68 34.04 32.32 30.51 29.48 17.72 16.34 14.73 12.86 10.66 9.31 ? ? ? ? ? ? 37.76 36.09 34.34 32.51 30.58 29.48 ? ? ? ? ? ? 208 a Numbers in italics indicate the source age of the MORB source, which is the age where the MORB source was derived from the Bulk Earth (BE) reservoir, bold numbers give the present-day isotopic composition of the MORB source. For details see appendix B. References are as follows: Hart and Staudigel [1989], Chauvel et al. [1992], Rehkämper and Hofmann [1997], and Hauri and Hart [1993]. MORB source is modeled by a single stage evolution starting from Bulk Earth (BE) at 4.55 Ga ago with m = 238U/204Pb = 8.2 and 232Th/238U = 3.8, similar to the approach taken in previous studies [e.g., Chauvel et al., 1992; Rehkämper and Hofmann, 1997; Roy-Barman and Allègre, 1995] (Table A.1). Hart and Staudigel [1989] model the Pb isotopic composition of the recycled oceanic crust by a two stage evolution model with a first stage equivalent to BE evolution between 4.55 and 4.0 Ga and a second stage evolution from BE at 4.0 27 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust to present-day MORB ( 206 Pb/ 204 Pb = 17.3, 208 Pb/204Pb = 37.3l; Table A.1 [Allègre et al., 1983a, 1983b]). Hart and Staudigel [1989] point out that no substantial difference in the Pb isotopic evolution of the MORB mantle results if a single stage evolution is preferred and Table A.1 shows that the two approaches differ only slightly. In the spread sheets, the Pb evolution is readily changed to a two stage evolution similar to that used by Hart and Staudigel [1989] and allowing for a variable age for the beginning of the second stage. However, it should be kept in mind that all these possible Pb evolution models are likely to be an oversimplification, because recent models predict an open system evolution of the mantle with respect to its U (Th) and Pb concentration, and consequently its Pb isotope composition [Elliott et al., 1999; Galer and O’Nions, 1985; White, 1993]. [68] The initial Sr, Nd, and Hf isotope composition of ancient oceanic crust is modeled by a two stage evolution of the MORB reservoir, with a first stage equivalent to BE evolution between 4.55 and XGa and a second stage evolution from BE at XGa to present-day MORB (87Sr/86Sr = 0.7027, 143Nd/ 144 Nd = 0.5132, and 176Hf/177Hf = 0.28335 (BE values today: 87Sr/86Sr = 0.705, 143Nd/144Nd = 0.512638, 176Hf/177Hf = 0.2828; see spread sheet ‘‘basalt + sed_recycling’’ and Table A.1). The beginning of the second stage can be chosen according to personal preference, but has been chosen to be 2 Ga for the examples shown in the Figures 1 and 4. The implications of different differentiation ages will be discussed and are readily investigated by choosing different source ages of the MORB source in the spread sheet ‘‘basalt + sed_recycling’’. Hauri and Hart [1993] have taken a similar approach but use a slightly different composition of the present-day BE and MORB reservoir (BE values today: 87Sr/86Sr = 0.7047, 143 Nd/144Nd = 0.512638; 176Hf/177Hf = 0.28288; MORB values today: 8 7 Sr/ 8 6 Sr = 0.7022, 143 Nd/144Nd = 0.5133, 176Hf/177Hf = 0.28355; see spread sheet ‘‘basalt-recycling’’ and Table A.1). Hart and Staudigel [1989] chose a source age of 4 Ga similar to their Pb isotope evolution (MORB reservoir today: 87 Sr/ 86 Sr = 0.7022, 143 Nd/144Nd = 0.5133, Table A.1 [Allègre et al., 10.1029/2001GC000223 1983a, 1983b]). Rehkämper and Hofmann [1997], on the other hand, use a single stage evolution starting from BE at 4.55 Ga ago and assume present-day MORB values of 87Sr/86Sr = 0.7025 and 143Nd/144Nd = 0.5132, similar to the approach taken by Chauvel et al. [1992] (Table A.1). [69] As shown in Table A.1, despite the different approaches, the initial Pb and Nd isotopic composition of the MORB reservoir at any given age differ only slightly. The initial Sr isotope composition, on the other hand, differs significantly as a function of the assumed source age of the MORB-source: the older the source age, the lower the 87Sr/86Sr of the MORB source at any given time. Furthermore, depending on the value chosen for the present-day MORB and BE reservoir, the initial 87Sr/86Sr ratio of the BE reservoir becomes greater than that of present-day MORB roughly between 1.7 and 2.2 Ga ago (for present-day MORB values of 0.7020 and 0.7027 and present-day BE of 0.7050), requiring relatively old source ages of the MORB source. This should be kept in mind when adjusting the source ages of the MORB source in the spread sheet ‘‘basalt + sed_recycling’’. [70] Furthermore, the Sr isotope composition of the MORB reservoir is affected by hydrothermal alteration processes at MOR [Staudigel et al., 1981b, 1995]. Based on the composition of fresh and hydrothermally altered crust [Kawahata et al., 1987, 1995; Staudigel et al., 1981a, 1996], it has been estimated that about 20–30% of the Sr in the altered basalts is replaced by seawater Sr [Hart and Staudigel, 1989; Rehkämper and Hofmann, 1997]. The reader has the option whether or not to allow for modification of the Sr isotope composition of ancient MORB, and can apply variable exchange rates and choose the isotopic composition of ancient seawater according to their own preference (see spread sheet ‘‘basalt + sed_recycling’’). For the examples shown in Figure 1, a 25% exchange rate as suggested by Rehkämper and Hofmann [1997] is adopted, and the Sr isotopic composition of ancient seawater is approximated using the seawater evolution curve by Shields and Veizer [2002] (see spread sheet ‘‘basalt + sed_recycling’’; see also [Rehkämper and Hofmann, 1997]). Chauvel et al. [1992] have taken a different approach by 28 of 33 Geochemistry Geophysics Geosystems 3 G 10.1029/2001GC000223 stracke et al.: recycling oceanic crust simply increasing the initial 87 Sr/86Sr of the recycled basalt by 0.5%, based on the 87Sr/86Sr ratios in fresh and altered basalts given by Kawahata et al. [1987]. Table A.2. Sediment Source Evolution: A Comparison of Different Approachesa Age (Ga) This study 87 B.2.2. Subducted Sediment [ 71 ] The initial isotopic composition of the recycled sediment is assumed to be equal to those of the sediment source (i.e. the continental crust (CC)) at the time of recycling. The reader can choose between three different types of evolution models for the sediment source in case of Sr, Nd, and Hf isotopes (see spread sheet ‘‘basalt + sed_recycling’’). (1) A simple BE evolution model. The BE evolution should provide a close estimate of the initial isotopic composition for old recycling ages (about 2Ga), but should be less appropriate for young recycling ages (2Ga). In any case, the BE evolution provides a maximum estimate of the initial Nd and Hf and a minimum estimate of the initial Sr isotopic composition. (2) A single stage evolution starting from BE at Xga ago to average present-day values of pelagic sediments (87Sr/86Sr 0.7200, 143Nd/144Nd 0.5122, 176Hf/177Hf 0.2824; the reader can adjust these values according to their own preference; see spread sheet ‘‘basalt + sed_recycling’’). Hart and Staudigel [1989] use a similar model where the sediment source is derived from BE 4 Ga ago and develops with average crustal composition to present-day values of average sediment (87Sr/86Sr 0.719, 143 Nd/144Nd 0.5103, 206Pb/204Pb 18.9, and 208 Pb/204Pb 38.9; Table A.2). This approach is also similar to that used by Rehkämper and Hofmann [1997] and Chauvel et al. [1992] with the difference that both studies use a single stage evolution from BE at 4.55 Ga ago to present-day average sediment values (87Sr/86Sr 0.7114 and 143 Nd/144Nd 0.5122; Chauvel et al. [1992] uses a similar approach but different values for modern sediments; Table A.2). (3) It can also be assumed that the sediment source is derived from BE at Xga ago and subsequently evolves with an average crustal composition [Rudnick and Fountain, 1995] until the time of recycling, in which case the initial isotopic composition of the sediment depends on its crustal residence time. 0 1 2 3 4 4.55 Hart and Staudigel Chauvel et al. Sr/86Sr (terrigenous/pelagic sediment) 0.71901 0.76838/0.73381 0.71140 0.71429 0.70874 0.70951 0.71076 0.70605 0.70465 0.70331 0.69970 0.70053 0.69900 143 0 1 2 3 4 4.55 Rehkämper and Hofmann Nd/144Nd 0.51034 0.50962 0.50889 0.50816 0.50740 0.51222 0.51102 0.50981 0.50860 0.50737 0.50670 206 0 1 2 3 4 4.55 18.70 17.07 15.16 12.93 10.54 9.35 18.81 17.22 15.36 13.18 10.64 Pb/204Pb 18.70 17.07 15.16 12.93 10.54 9.35 208 0 1 2 3 4 4.55 a 38.63 36.76 34.80 32.74 30.63 29.52 38.84 36.89 34.85 32.71 30.45 Pb/204Pb 38.63 36.76 34.80 32.74 30.63 29.52 18.70 17.07 15.16 12.93 10.54 9.35 38.63 36.76 34.80 32.74 30.63 29.52 See notes in Table A.1. [72] There are significant differences in the initial Sr, Nd and Hf isotopic composition of the sediment source depending on the preferred evolution model. The effects of different initial isotopic composition of the sediment reservoir can readily be investigated by choosing between the three different evolution models in the spread sheet ‘‘basalt + sed_recycling’’. Compared to BE values, both the single stage evolution and evolution in the CC lead to lower initial Nd and Hf and to higher Sr isotope ratios for similar source ages. These different initial isotope ratios also lead to lower presentday Nd and Hf and higher Sr isotope ratios in the bulk subducted crust. The slope of the mixing line between pure oceanic crust and sediment, on the other hand does not change as it depends only on the compositional difference between oceanic crust and sediment. Therefore, the main effect is to 29 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust develop more enriched (crustal-like) isotopic compositions for a given recycling age. This effect is most pronounced for Sr isotopes but is less significant for Nd and Hf isotopes (see spread sheet ‘‘basalt + sed_recycling’’). [73] Contrary to the Sr, Nd, and Hf isotopes, the Pb isotopic evolution of the CC is well recorded in galenas and has been reproduced by the Stacey and Kramers [1975] model. Therefore, the Stacey and Kramers [1975] model is used here to estimate the Pb isotopic evolution of the sediment reservoir, analogous to Chauvel et al. [1992] and Rehkämper and Hofmann [1997] (Table A.2). Again, two-stage models similar to those chosen for Sr, Nd and Hf isotopes are readily implemented in the spread sheet ‘‘basalt + sed_recycling’’. B.3. Trace Element Composition of the Recycled Crust B.3.1. Oceanic Crust [74] In principle, any plausible basalt composition can be used for the modeling. A variety of different estimates of average MORB [Hofmann, 1988; Sun and McDonough, 1989], present and ancient gabbros [Hart et al., 1999; Zimmer et al., 1995] and altered oceanic crust [Staudigel et al., 1995, 1996] is used in the examples presented in Figure 1 and listed in the spread sheet ‘‘basalt + sed_recycling’’. Compared to average MORB [Hofmann, 1988; Sun and McDonough, 1989], hydrothermally altered oceanic crust is characterized by an increase in alkali element (Cs, Rb, K) and U concentrations, whereas the rare earth elements (REE) and high field strength elements (HFSE) remain relatively unchanged [Staudigel et al., 1995, 1996] (see Table 1). Previous estimates of the altered oceanic crust [Staudigel et al., 1981a, 1981b, 1983] used by Hart and Staudigel [1989] have slightly lower U/Pb, Th/ Pb and Rb/Sr ratios but similar Sm/Nd and Lu/Hf ratios. The gabbros [Hart et al., 1999; Zimmer et al., 1995] have broadly similar composition to the average MORBs [Hofmann, 1988; Sun and McDonough, 1989] but have lower U/Pb and Th/Pb ratios. [75] Chauvel et al. [1992] and Rehkämper and Hofmann [1997] and Hart and Staudigel [1989] 10.1029/2001GC000223 all use a single composition of altered oceanic crust. Hart and Staudigel [1989] use an estimate based on the studies of Staudigel et al. [e.g., Staudigel et al., 1981a, 1981b, 1983], whereas Chauvel et al. [1992] and Rehkämper and Hofmann estimated the composition of the altered oceanic crust by modifying average MORB [Hofmann, 1988]. Chauvel et al. [1992] decrease the Pb and increase the U concentration by 56% and 12%, respectively; other elements remain unchanged, which is at odds with the increase in alkali elements (Cs, Rb, K) observed in the altered oceanic crust [Staudigel et al., 1995; 1996]. Rehkämper and Hofmann [1997] increase the U and Rb concentration by 15% and 40%, respectively, and decrease the Pb concentration by 35%. Compared to the altered oceanic crust [Staudigel et al., 1995; 1996] the estimates of Chauvel et al. [1992] and Rehkämper and Hofmann result in significantly lower Rb/Sr, U/Pb, and Th/Pb ratios but have broadly similar Sm/Nd and Lu/Hf ratios. B.3.2. Recycled Sediments [76] Plank and Langmuir’s [1998] estimates of the bulk composition of sediment approaching subduction zones are used in this study in conjunction with Johnson’s and Plank’s [2000] estimates of sub-arc modification. [77] Hart and Staudigel [1989] used the compositions of recent pelagic sediments given by White et al. [1985], whereas Chauvel et al. [1992] and Rehkämper and Hofmann [1997] used averages of the terrigenous and pelagic sediments given by Othman et al. [1989] (which are also listed in the spread sheet ‘‘basalt + sed_recycling’’). Because the actual composition of subducted sediments represents a composite of sediments of different lithologies, the estimates of Plank and Langmuir [1998] can be significantly different from those of a single lithology. Acknowledgments [78] The editors B. White and H. Staudigel and two anonymous reviewers are thanked for thoughtful comments which helped to improve the presentation of the manuscript and the documentation of the modeling. A.S. was in part supported by a HSP-III doctoral fellowship by the German Academic 30 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust Exchange Service (DAAD). This work was partly supported by NSF award EAR-0124965 to V.S. References Allègre, C. J., S. R. Hart, and J. F. Minster, Chemical structure and evolution of the mantle and the continents determined by inversion of Nd and Sr isotopic data I, Theoretical models, Earth Planet. Sci. Lett., 66, 177 – 190, 1983a. Allègre, C. J., S. R. Hart, and J. F. Minster, Chemical structure and evolution of the mantle and the continents determined by inversion of Nd and Sr isotopic data, II, Numerical experiments and discussion, Earth Planet. Sci. Lett., 66, 191 – 213, 1983b. Armstrong, R. L., A model for the evolution of strontium and lead isotopes in a dynamic Earth, Rev. Geophys., 6, 175 – 199, 1968. Ayers, J. C., Trace element modeling of aqueous fluid-peridotite interaction in the mantle wedge of subduction zones, Contrib. Mineral. Petrol., 132, 390 – 404, 1998. Ayers, J. C., S. K. Dittmer, and G. D. Layne, Partitioning of elements between peridotite and H2O at 2.0 – 3.0 GPa and 900 – 1100C, and application to models of subduction zone processes, Earth Planet. Sci. Lett., 150, 381 – 398, 1997. Barling, J., and S. L. Goldstein, Extreme isotopic variations in Heard Island lavas and the nature of mantle reservoirs, Nature, 348, 59 – 62, 1990. Beattie, P., The generation of uranium series disequilibria by partial melting of spinel peridotite: Constraints from partitioning studies, Earth Planet. Sci. Lett., 117, 379 – 391, 1993. Ben Othman, D., W. M. White, and J. Patchett, The geochemistry of marine sediments, island arc magma genesis, and crust – mantle recycling, Earth Planet. Sci. Lett., 94, 1 – 21, 1989. Bennett, V. C., T. M. Esat, and M. D. Norman, Two mantleplume components in Hawaiian picrites inferred from correlated Os-Pb isotopes, Nature, 381, 221 – 224, 1996. Bennett, V. C., M. D. Norman, and M. O. Garcia, Rhenium and platinum group element abundances correlated with mantle source components in Hawaiian picrites: Sulphides in the plume, Earth Planet. Sci. Lett., 183, 513 – 526, 2000. Bizimis, M., V. J. M. Salters, and E. Bonatti, Trace and REE content of clinopyroxenes from supra-subduction zone peridotites: Implications for melting and enrichment processes in island arcs, Chem. Geol., 165, 67 – 85, 2000. Blichert-Toft, J., F. A. Frey, and F. Albarede, Hf isotope evidence for pelagic sediments in the source of Hawaiian basalts, Science, 285, 879 – 882, 1999. Brandon, A. D., M. D. Norman, R. J. Walker, and J. W. Morgan, 186Os-187Os systematics of Hawaiian picrites, Earth Planet. Sci. Lett., 174, 25 – 42, 1999. Brandon, A. D., R. J. Walker, J. W. Morgan, M. D. Norman, and H. Prichard, Coupled 186Os and 187Os evidence for coremantle interaction, Science, 280, 1570 – 1573, 1998. Brenan, J. M., H. F. Shaw, D. L. Phinney, and F. J. Ryerson, Rutile-aqueous fluid partitioning of Nb, Ta, Hf, Zr, U and 10.1029/2001GC000223 Th: Implications for high-field strength element depletions in island-arc basalts, Earth Planet. Sci. Lett., 128, 327 – 339, 1994. Brenan, J. M., H. F. Shaw, F. J. Ryerson, and D. L. Phinney, Mineral-aqueous fluid partitioning of trace-elements at 900C and 2.0 Gpa: Constraints on the trace-element chemistry of mantle and deep-crustal fluids, Geochim. Cosmochim. Acta, 59, 3331 – 3350, 1995. Chaffey, D. J., R. A. Cliff, and B. M. Wilson, Characterization of the St. Helena magma source, in Magmatism in the Ocean Basins, edited by A. D. Saunders and M. J. Norry, Geol. Soc. Spec. Publ., 42, 257 – 276, 1989. Chase, C. G., Oceanic island lead: Two-stage histories and mantle evolution, Earth Planet. Sci. Lett., 52, 277 – 284, 1981. Chauvel, C., A. W. Hofmann, and P. Vidal, HIMU-EM: The French-Polynesian Connection, Earth Planet. Sci. Lett., 110, 99 – 119, 1992. Chauvel, C., W. McDonough, G. Guille, R. Maury, and R. Duncan, Contrasting old and young volcanism in Rurutu Island, Austral chain, Chem. Geol., 139, 125 – 143, 1997. Eisele, J., M. Sharma, J. G. Galer, J. Blichert-Toft, C. W. Devey, and A. W. Hofmann, The role of sediment recycling in EMI inferred from Os, Pb, Hf, Nd, Sr isotope and trace element systematics in the Pitcairn hotspot, Earth Planet. Sci. Lett., 196, 197 – 212, 2002. Elliott, T., T. Plank, A. Zindler, W. White, and B. Bourdon, Element transport from slab to volcanic front at the Mariana arc, J. Geophys. Res., 102, 14,991 – 15,019, 1997. Elliott, T., A. Zindler, and B. Bourdon, Exploring the Kappa Conundrum: The role of recycling in the lead isotope evolution of the mantle, Earth Planet. Sci. Lett., 169, 129 – 145, 1999. Galer, S. J. G., and R. K. O’Nions, Residence time of thorium, uranium and lead in the mantle with implications for mantle convection, Nature, 316, 778 – 782, 1985. Gill, J. B., and R. W. Williams, Th isotope and U-series studies of subduction-related volcanic rocks, Geochim. Cosmochim. Acta, 54, 1427 – 1442, 1990. Grand, S. P., and S. Widiyantoro, Global seismic tomography: a snapshot of convection in the Earth, Geol. Soc. Am. Today, 7, 1 – 7, 1997. Halliday, A. N., A. P. Dickin, and A. E. Fallick, Mantle dynamics: A Nd, Sr, Pb and O isotopic study of the Cameroon Line volcanic chain, J. Petrol., 29, 181 – 211, 1988. Halliday, A. N., D. C. Lee, S. Tommasini, G. R. Davies, C. R. Paslick, J. G. Fitton, and D. E. James, Incompatible traceelements in OIB and MORB and source enrichment in the sub-oceanic mantle, Earth Planet. Sci. Lett., 133, 379 – 395, 1995. Hart, S. R., Heterogeneous mantle domains: Signatures, genesis and mixing chronologies, Earth Planet. Sci. Lett., 90, 273 – 296, 1988. Hart, S. R., J. Blusztajn, H. J. Dick, P. S. Meyer, and K. Muehlenbachs, The fingerprint of seawater circulation in a 500-meter section of ocean crust gabbros, Geochim. Cosmochim. Acta, 63, 4059 – 4080, 1999. 31 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust Hart, S. R., and T. Dunn, Experimental Cpx melt partitioning of 24 trace-elements, Contrib. Mineral. Petrol., 113, 1 – 8, 1993. Hart, S. R., and H. Staudigel, Isotopic characterization and identification of recycled components, in Crust/Mantle Recycling at Convergence Zones, edited by S. R. Hart and L. Gulen, pp. 15 – 28, Kluwer Acad., Norwell, Mass., 1989. Hauri, E., T. P. Wagner, and T. L. Grove, Experimental and natural partitioning of Th, U, Pb and other trace elements between garnet, clinopyroxene and basaltic melts, Chem. Geol., 117, 149 – 166, 1994. Hauri, E. H., and S. R. Hart, Re-Os Isotope systematics of HIMU and EMII oceanic island basalts from the South-Pacific Ocean, Earth Planet. Sci. Lett., 114, 353 – 371, 1993. Hauri, E. H., J. C. Lassiter, and D. J. DePaolo, Osmium isotope systematics of drilled lavas from Mauna Loa, Hawaii, J. Geophys. Res., 101, 11,793 – 11,806, 1996. Hemond, C., C. W. Devey, and C. Chauvel, Source compositions and melting processes in the society and austral plumes (South-Pacific Ocean): Element and isotope (Sr, Nd, Pb, Th) geochemistry, Chem. Geol., 115, 7 – 45, 1994. Hofmann, A. W., Chemical differentiation of the Earth: The relationship between mantle, continental-crust, and oceaniccrust, Earth Planet. Sci. Lett., 90, 297 – 314, 1988. Hofmann, A. W., Mantle geochemistry: The message from oceanic volcanism, Nature, 385, 219 – 229, 1997. Hofmann, A. W., and W. M. White, Mantle plumes from ancient oceanic-crust, Earth Planet. Sci. Lett., 57, 421 – 436, 1982. Johnson, K. T. M., Experimental determination of partition coefficients for rare earth and high-field-strength elements between clinopyroxene, garnet, and basaltic melt at high pressures, Contrib. Mineral. Petrol., 133, 60 – 68, 1998. Johnson, M. C., and T. Plank, Dehydration and melting experiments constrain the fate of subducted sediments, Geochem. Geophys. Geosys., 1, Paper number 1999GC000014, 2000. Kawahata, H., M. Kusakabe, and Y. Kikuchi, Strontium, oxygen, and hydrogen isotope geochemistry of hydrothermally altered and weathered rocks in DSDP hole 504B, Costa Rica rift, Earth Planet. Sci. Lett., 85, 343 – 355, 1987. Kennedy, A. K., G. E. Lofgren, and G. J. Wasserburg, An experimental study of trace element partitioning between olivine, orthopyroxene and melt in chondrules: equilibrium values and kinetic effects, Earth Planet. Sci. Lett., 115, 177 – 195, 1993. Keppler, H., Constraints from partitioning experiments on the composition of subduction-zone fluids, Nature, 380, 237 – 240, 1996. Kogiso, T., Y. Tatsumi, and S. Nakano, Trace element transport during dehydration processes in the subducted oceanic crust, 1, Experiments and implications for the origin of ocean island basalts, Earth Planet. Sci. Lett., 148, 193 – 205, 1997. Lassiter, J., and E. Hauri, Osmium-isotope variations in Hawaiin lavas: evidence for recycled oceanic lithosphere in the Hawaiian plume, Earth Panet. Sci. Lett., 164, 483 – 496, 1998. 10.1029/2001GC000223 Le Roex, A. P., R. A. Cliff, and B. J. I. Adair, Tristan-DaCunha, South-Atlantic: Geochemistry and petrogenesis of a basanite phonolite lava series, J. Petrol., 31, 779 – 812, 1990. McCulloch, M. T., and J. A. Gamble, Geochemical and geodynamical constraints on subduction zone magmatism, Earth Planet. Sci. Lett., 102, 358 – 374, 1991. McDonough, W. F., and S.-S. Sun, The composition of the Earth, Chem. Geol., 120, 223 – 253, 1995. Nakamura, Y., and M. Tatsumoto, Pb, Nd, and Sr isotopic evidence for a multicomponent source for rocks of CookAustral Islands and heterogeneities of mantle plumes, Geochim. Cosmochim. Acta, 52, 2909 – 2924, 1988. Newsom, H. E., W. M. White, K. P. Jochum, and A. W. Hofmann, Siderophile and chalcophile element abundances in oceanic basalts, pb-isotope evolution and growth of the Earths core, Earth Planet. Sci. Lett., 80, 299 – 313, 1986. Palacz, Z. A., and A. D. Saunders, Coupled trace element and isotope enrichment in the Cook-Austral-Samoa islands, southwest Pacific, Earth Planet. Sci. Lett., 79, 270 – 280, 1986. Peucker-Ehrenbrink, B., Re-Os systematics and PGE-budgets of contintental and oceanic crust, paper presented at GERM workshop, Nat. Sci. Foundation, Arlington, Va., 2001. Plank, T., and C. H. Langmuir, The chemical composition of subducting sediment and its consequences for the crust and mantle, Chem. Geol., 145, 325 – 394, 1998. Rehkämper, M., and A. W. Hofmann, Recycled ocean crust and sediment in Indian Ocean MORB, Earth Planet. Sci. Lett., 147, 93 – 106, 1997. Reisberg, L., A. Zindler, F. Marcantonio, W. White, D. Wyman, and B. Weaver, Os Isotope Systematics in Ocean Island Basalts, Earth Planet. Sci. Lett., 120, 149 – 167, 1993. Roy-Barman, M., and C. J. Allègre, 187Os/186Os in oceanic island basalts: Tracing oceanic crust recycling in the mantle, Earth Planet. Sci. Lett., 129, 145 – 161, 1995. Rudnick, R. L., and D. M. Fountain, Nature and composition of the continental crust: A lower crustal perspective, Rev. Geophys., 33, 267 – 309, 1995. Salters, V. J. M., and W. M. White, Hf isotope constraints on mantle evolution, Chem. Geol., 145, 447 – 460, 1998. Shaw, D. M., Trace element fractionation during anatexis, Geochim. Cosmochim. Acta, 34, 237 – 243, 1970. Shields, G., and J. Veizer, Precambrian marine carbonate isotope database: Version 1.1, Earth Planet. Sci. Lett., 3, 12, 2002. Shirey, S. B., and R. J. Walker, The Re-Os system in cosmochemistry and high-temperature geochemistry, Ann. Rev. Earth Planet. Sci., 26, 423 – 500, 1998. Stacey, J. S., and J. D. Kramers, Approximation of terrestrial lead isotope evolution by a two-stage model, Earth Planet. Sci. Lett., 26, 207 – 221, 1975. Stalder, R., S. F. Foley, G. P. Brey, and I. Horn, Mineral-aqueous fluid partitioning of trace elements at 900 – 1200C and 3.0 – 5.7GPa: New experimental data for garnet, clinopyroxene, and rutile, and implications for mantle metasomatism, Geochim. Cosmochim. Acta, 62, 1781 – 1801, 1998. 32 of 33 Geochemistry Geophysics Geosystems 3 G stracke et al.: recycling oceanic crust Staudigel, H., G. R. Davies, S. R. Hart, K. M. Marchant, and B. M. Smith, Large scale isotopic Sr, Nd and O isotopic anatomy of altered oceanic crust: DSDP/ODP sites 417/ 418, Earth Planet. Sci. Lett., 130, 169 – 185, 1995. Staudigel, H., and S. R. Hart, Alteration of basaltic glass: Mechanisms and significance for the oceanic crust-seawater budget, Geochim. Cosmochim. Acta, 47, 337 – 350, 1983. Staudigel, H., S. R. Hart, and S. H. Richardson, Alteration of the oceanic crust: processes and timing, Earth Planet. Sci. Lett., 52, 311 – 327, 1981a. Staudigel, H., K. Muelhenbachs, S. H. Richardson, and S. R. Hart, Agents of low temperature ocean crust alteration, Contrib. Mineral. Petrol., 77, 150 – 157, 1981b. Staudigel, H., T. Plank, W. M. White, and H. U. Schmincke, Geochemical fluxes during seafloor alteration of the basaltic upper crust: DSDP sites 417 and 418, in Subduction: Top to Bottom, Geophys. Monogr. Ser., vol. 96, edited by G. E. Bebout, D. W. Scholl, S. H. Kirby, and J. P. Platt, pp. 19 – 38, AGU, Washington, D.C., 1996. Sun, S.-S., and W. F. McDonough, Chemical and isotopic systematics of oceanic basalts: implications for mantle composition and processes, in Magmatism in the Ocean Basins, edited by A. D. Saunders and M. J. Norry, Geol. Soc. Spec. Publ., 42, 313 – 345, 1989. Tatsumi, Y., Migration of fluid phases and genesis of basalt magmas in subduction zones, J. Geophys. Res., 94, 4697 – 4707, 1989. Tatsumi, Y., and Y. Kogiso, Trace element transport during dehydration processes in the subducted oceanic crust, 2, Origin of chemical and physical characteristics in arc magmatism, Earth Planet. Sci. Lett., 148, 207 – 221, 1997. Tatsumoto, M., R. J. Knight, and C. J. Allègre, Time differences in the formation of meteorites as determined from the ratio of lead-207 to lead-206, Science, 180, 1278 – 1283, 1973. Turner, S., and C. Hawkesworth, Constraints on flux rates and mantle dynamics beneath island arcs from Tonga-Kermadec lava geochemistry, Nature, 389, 568 – 573, 1997. Turner, S., C. Hawkesworth, N. Rogers, J. Bartlett, T. Worthington, J. Hergt, J. Pearce, and I. Smith, U-238-Th-230 disequilibria, magma petrogenesis, and flux rates beneath the depleted Tonga-Kermadec island arc, Geochim. Cosmochim. Acta, 61, 4855 – 4884, 1997. Turner, S., C. Hawkesworth, P. van Calsteren, E. Heath, R. Macdonald, and S. Black, U-series isotopes and destructive plate margin magma genesis in the Lesser Antilles, Earth Planet. Sci. Lett., 142, 191 – 207, 1996. van der Hilst, R., S. Widiyantoro, and E. R. Engdahl, Evidence for deep mantle circulation from global tomography, Nature, 386, 578 – 584, 1997. Veizer, J., and W. Compston, 87Sr/86Sr in Precambrian carbonates as an index of crustal evolution, Geochim. Cosmochim. Acta, 40, 905 – 915, 1976. 10.1029/2001GC000223 Vidal, P., C. Chaucel, and R. Brousse, Large mantle heterogeneity beneath French Polenesia, Nature, 307, 536 – 538, 1984. Walker, R. J., J. W. Morgan, and M. Horan, Osmium 187 enrichments in some plumes: a consequence of core-mantle interaction?, Science, 269, 819 – 822, 1995. Weaver, B. L., The origin of ocean island basalt end-member compositions: Trace element and isotopic constraints, Earth Planet. Sci. Lett., 104, 381 – 397, 1991. Weis, D., F. A. Frey, H. Leyrit, and I. Gautier, Kerguelen Archipelago: Geochemical and isotopic study of the Southast Province lavas, Earth Planet. Sci. Lett., 118, 101 – 119, 1993. White, R. S., D. McKenzie, and A. K. O’Nions, Oceanic crustal thickness from seismic measurements and rare earth element inversions, J. Geophys. Res., 97, 19,683 – 19,715, 1992. White, W., and R. Duncan, Geochemistry and geochronology of the Society Islands: New evidence for deep mantle recycling, Isotope Studies of Crust Mantle Evolution, edited by S. R. Hart and A. Basu, AGU, Washington, D.C., 1995. White, W. M., Sources of oceanic basalts: Radiogenic isotopic evidence, Geology, 13, 115 – 118, 1985. White, W. M., U-238/Pb-204 in MORB and open system evolution of the depleted mantle, Earth Planet. Sci. Lett., 115, 211 – 226, 1993. White, W. M., B. Dupré, and P. Vidal, Isotope and trace element geochemistry of sediments from the Barbados Ridge-Demerara Plain region, Atlantic Ocean, Geochim. Cosmochim. Acta, 49, 1875 – 1886, 1985. Widom, E., K. A. Hoernle, S. B. Shirey, and H. U. Schmincke, Os isotope systematics in the Canary Islands and Madeira: Lithospheric contamination and mantle plume signatures, J. Petrol., 40, 279 – 296, 1999. Widom, E., and S. B. Shirey, Os isotope systematics in the Azores: Implications for mantle plume sources, Earth Planet. Sci. Lett., 142, 451 – 465, 1996. Woodhead, J. D., and C. W. Devey, Geochemistry of the pitcairn seamounts, 1, Source character and temporal trends, Earth Planet. Sci. Lett., 116, 81 – 99, 1993. Woodhead, J. D., and M. T. McCulloch, Ancient seafloor signals in pitcairn-island lavas and evidence for large-amplitude, small length-scale mantle heterogeneities, Earth Planet. Sci. Lett., 94, 257 – 273, 1989. Wright, E., and W. M. White, The Origin of Samoa: New evidence from Sr, Nd, and Pb isotopes, Earth Planet. Sci. Lett., 81, 151 – 162, 1987. Zimmer, M., A. Kroener, K. P. Jochum, T. Reischmann, and W. Todt, The Gabal Gerf complex: A precambrian N-MORB ophiolite in the Nubian shield, NE Africa, Chem. Geol., 123, 29 – 51, 1995. Zindler, A., and S. Hart, Chemical Geodynamics, Ann. Rev. Earth Planet. Sci., 14, 493 – 571, 1986. Zindler, A., and E. Jagoutz, Mantle Cryptology, Geochim. Cosmochim. Acta, 52, 319 – 333, 1988. 33 of 33