Bonding Energy, Lewis Structures, and VSEPR Worksheet Bond
advertisement
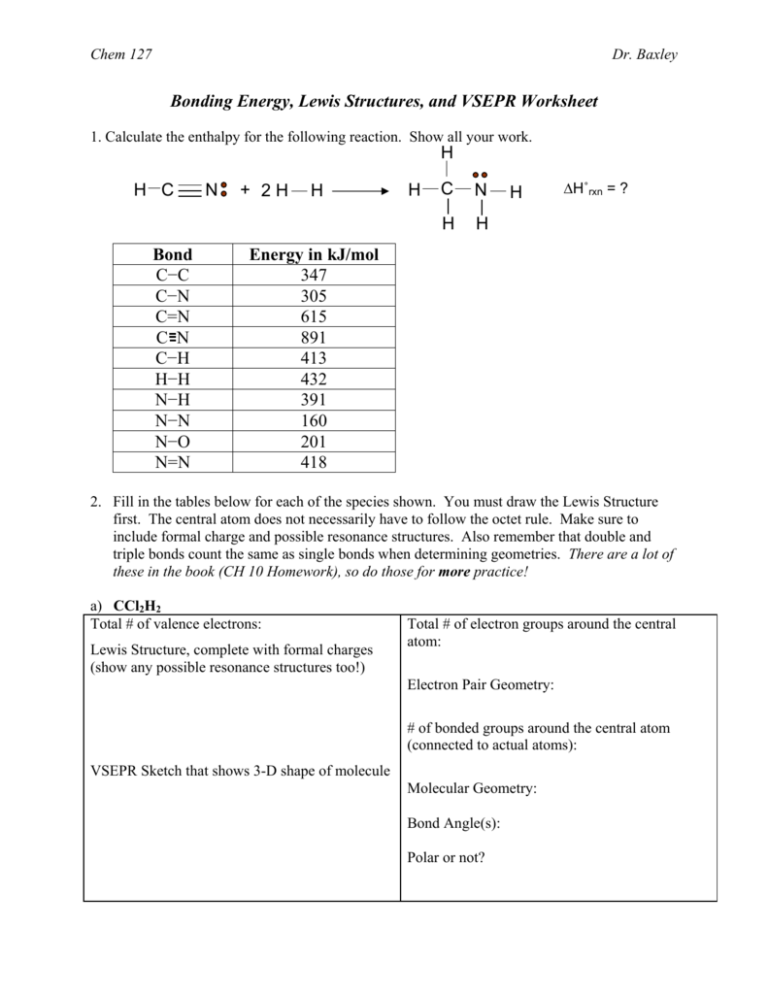
Chem 127 Dr. Baxley Bonding Energy, Lewis Structures, and VSEPR Worksheet 1. Calculate the enthalpy for the following reaction. Show all your work. H H C Bond C−C C−N C=N C N C−H H−H N−H N−N N−O N=N N + 2H H H C N H H H ΔH˚rxn = ? Energy in kJ/mol 347 305 615 891 413 432 391 160 201 418 2. Fill in the tables below for each of the species shown. You must draw the Lewis Structure first. The central atom does not necessarily have to follow the octet rule. Make sure to include formal charge and possible resonance structures. Also remember that double and triple bonds count the same as single bonds when determining geometries. There are a lot of these in the book (CH 10 Homework), so do those for more practice! a) CCl2H2 Total # of valence electrons: Lewis Structure, complete with formal charges (show any possible resonance structures too!) Total # of electron groups around the central atom: Electron Pair Geometry: # of bonded groups around the central atom (connected to actual atoms): VSEPR Sketch that shows 3-D shape of molecule Molecular Geometry: Bond Angle(s): Polar or not? Chem 127 Dr. Baxley b) ClNO Total # of valence electrons: Lewis Structure, complete with formal charges (show any possible resonance structures too!) Total # of electron groups around the central atom: Electron Pair Geometry: # of bonded groups around the central atom (connected to actual atoms): VSEPR Sketch that shows 3-D shape of molecule Molecular Geometry: Bond Angle(s): Polar or not? c) PF6− Total # of valence electrons: Lewis Structure, complete with formal charges (show any possible resonance structures too!) Total # of electron groups around the central atom: Electron Pair Geometry: # of bonded groups around the central atom (connected to actual atoms): VSEPR Sketch that shows 3-D shape of molecule Molecular Geometry: Bond Angle(s): Polar or not? Chem 127 d) SbF4− Total # of valence electrons: Lewis Structure, complete with formal charges (show any possible resonance structures too!) Dr. Baxley Total # of electron groups around the central atom: Electron Pair Geometry: # of bonded groups around the central atom (connected to actual atoms): VSEPR Sketch that shows 3-D shape of molecule Molecular Geometry: Bond Angle(s): Polar or not? e) NO2− Total # of valence electrons: Lewis Structure, complete with formal charges (show any possible resonance structures too!) Total # of electron groups around the central atom: Electron Pair Geometry: # of bonded groups around the central atom (connected to actual atoms): VSEPR Sketch that shows 3-D shape of molecule Molecular Geometry: Bond Angle(s): Polar or not? Chem 127 Dr. Baxley Answer Key 1. –158 kJ 2. a. electron pair geometry: tetrahedral molecular geometry: tetrahedral bond angle: 109.5º polar Cl H C Cl H b. electron pair geometry: trigonal planar molecular geometry: bent bond angle: <120º polar Cl N O c. electron pair geometry: octahedral molecular geometry: octahedral bond angle: 90º nonpolar d. electron pair geometry: trigonal bipyramidal molecular geometry: see-saw bond angle: 90º & <120º polar F F S F F e. electron pair geometry: trigonal planar molecular geometry: bent bond angle: <120º polar O N O O N O





