VSEPR: Valence Shell Electron Pair Repulsion Theory
advertisement
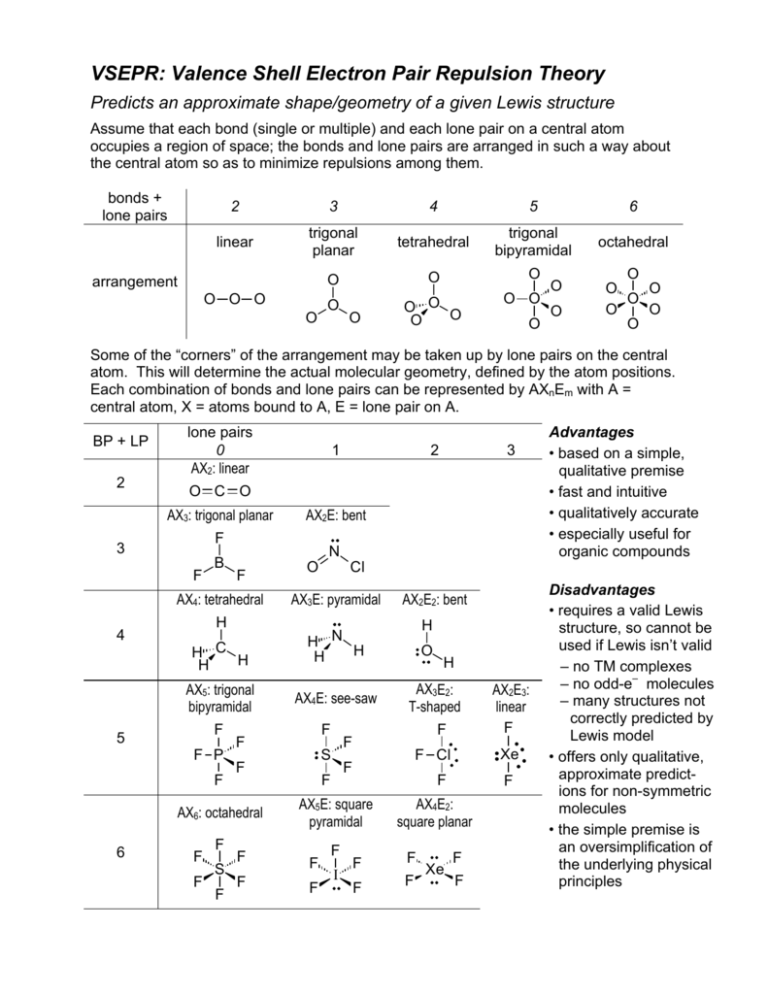
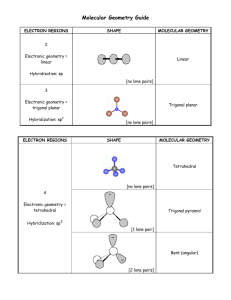
VSEPR: Valence Shell Electron Pair Repulsion Theory Predicts an approximate shape/geometry of a given Lewis structure Assume that each bond (single or multiple) and each lone pair on a central atom occupies a region of space; the bonds and lone pairs are arranged in such a way about the central atom so as to minimize repulsions among them. bonds + lone pairs 2 3 4 5 6 linear trigonal planar tetrahedral trigonal bipyramidal octahedral O O O O O O O O arrangement O O O O O O O O O O O O O O O O O Some of the “corners” of the arrangement may be taken up by lone pairs on the central atom. This will determine the actual molecular geometry, defined by the atom positions. Each combination of bonds and lone pairs can be represented by AXnEm with A = central atom, X = atoms bound to A, E = lone pair on A. BP + LP 2 lone pairs 0 AX2: linear 3 AX2E: bent F 3 F B N F AX4: tetrahedral H H C H H AX5: trigonal bipyramidal F 5 F P F F F F S F O F F AX2E2: bent H H N H H AX4E: see-saw F F F I F F AX4E2: square planar F F F F Xe AX2E3: linear F Xe F Cl AX5E: square pyramidal F H AX3E2: T-shaped F F F O F S F Advantages • based on a simple, qualitative premise • fast and intuitive • qualitatively accurate • especially useful for organic compounds Cl AX3E: pyramidal F AX6: octahedral 6 2 O C O AX3: trigonal planar 4 1 F F Disadvantages • requires a valid Lewis structure, so cannot be used if Lewis isn’t valid – no TM complexes – no odd-e– molecules – many structures not correctly predicted by Lewis model • offers only qualitative, approximate predictions for non-symmetric molecules • the simple premise is an oversimplification of the underlying physical principles How to Determine a VSEPR Structure 1) Draw the Lewis Structure 2) Count the bonds and lone pairs about the central atom (note that multiple bonds are one region of electron density) 3) Determine the electron pair arrangement (both bond pairs and lone pairs) 4) Position the atoms and lone pairs so as to minimize lone pair repulsions 5) Name the molecular geometry based on the position of the atoms Examples 180º BeF2 AX2: linear arrangement linear geometry F Be F F AX3: trigonal planar arrangement trigonal planar geometry 120º BF3 B F F H 109.5º CH4 AX4: tetrahedral arrangement tetrahedral geometry H C H H 90º F PF5 F P 120º F F AX5: trigonal bipyramidal arrangement trigonal bipyramidal geometry F F F F S F F 90º F SF6 AX6: octahedral arrangement octahedral geometry O O NO3– N O O N AX3, regardless of resonance: trigonal planar O O O O N • multiple bonds count as a single density region! • resonance forms do not affect VSEPR! O All AXn molecules have identical electron pair arrangement and molecular geometry! More Examples H 109.5º CH4 NH3 H2O H C H H H N H H P 120º Two “corners” are lone pairs! F F F S F F F F Cl F F XeF2 AX5: trigonal bipyramidal arrangement trigonal bipyramidal geometry F F ClF3 One “corner” is a lone pair! F F SF4 AX3E: tetrahedral arrangement trigonal pyramidal geometry AX2E2: tetrahedral arrangement bent geometry H O H 90º PF5 AX4: tetrahedral arrangement tetrahedral geometry Xe F AX4E: trigonal bipyramidal arrangement “see-saw” geometry First LP: equatorial AX3E2: trigonal bipyramidal arrangement “T-shape” geometry Second LP: also equatorial AX2E3: trigonal bipyramidal arrangement linear geometry All LP equatorial in trigonal bipyramidal! Which corners do the lone pairs occupy? 1) tighter angle = more repulsion: 90º > 120º > 180º ⎯⎯⎯⎯⎯⎯⎯⎯⎯→ less repulsion 2) lone pairs need more room than bond pairs: interaction: LP-LP > LP-BP > BP-BP ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯→ less repulsion LPs occupy positions that give greatest LP-LP and LP-BP angles

![Which is the correct Lewis structure for the nitrate ion, [NO3]– ? a) b](http://s3.studylib.net/store/data/008121614_1-3f41411d21eef682c95d3c7778684719-300x300.png)