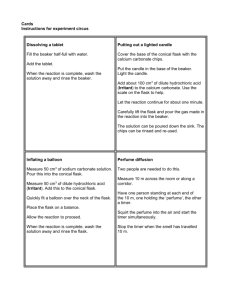
ubco course pack chem 111/113 121/123 laboratory manual
advertisement

UBCO COURSE PACK CHEM 111/113 121/123 LABORATORY MANUAL EYE PROTECTION MUST BE USED IN ALL LABORATORIES Winter 2012/2013 CHEMISTRY 111/113 and 121/123 LABORATORY MANUAL General Regulations And Procedures ............................................................................................. 3 The Laboratory Notebook And Reports ....................................................................................... 10 Significant Figures ........................................................................................................................ 13 Errors, Accuracy And Precision ................................................................................................... 14 General Laboratory Techniques .................................................................................................... 18 Instruments .................................................................................................................................... 21 Drawing A Graph .......................................................................................................................... 24 Lab A. Using Excel for Graphing ................................................................................................. 27 Lab B. The Determination of Density and the Application of Uncertainties ............................... 29 Lab C. Water of Hydration: Determination of an Empirical Formula .......................................... 37 Lab E. Spectrophotometric Determination of Iron ....................................................................... 47 Lab F. Standardization of a Sodium Thiosulphate Solution ......................................................... 57 Lab G. Volumetric Analysis for Vitamin C (C6H8O6) ................................................................. 67 Lab K. Determination of Water Hardness .................................................................................... 75 Lab W. The Molar Volume of Nitrogen ....................................................................................... 81 Lab L. Polymer Chemistry ............................................................................................................ 91 Lab N. Thermochemistry (Calorimetry) ..................................................................................... 101 Lab P. The Determination of the Solubility Product Constant for Calcium Iodate .................... 111 Lab Q. Chemical Equilibrium: Le Châtélier's Principle ............................................................. 117 Lab S. Acids and Bases: Titrations ............................................................................................. 127 Lab U. Kinetics: The Investigation of the Rate of Chemical Reaction ...................................... 135 Lab Z. The Preparation of Acetanilide ....................................................................................... 147 Appendix ..................................................................................................................................... 153 Chem 111 or 121 (circle one) Section Year/Term________ No student will be allowed in the lab without agreeing to and signing the statement below. Please hand it directly to your instructor who will then check off your name on the class list. To ensure that Chem 111/121 labs are carried out safely, I, _________________________ (print name) WILL read and complete the prelab for each lab, and make sure I understand the correct and safe use of equipment and lab techniques. I will comply with all safety instructions given in the lab manual and by the TA and I will report to the TA any and all accidents or injuries. I furthermore agree to look up all hazards of every substance which I will come in contact with for each lab. I also understand that while in the lab I need to wear appropriate eye protection and a cotton lab coat at all times. I will ask questions about any safety issues that I am unsure about. Signed, ______________________________ ________ ___, 201___ Signature ___________________________ Student Number Month Day Year Chem 113 or 123 (circle one) Section Year/Term_________ No student will be allowed in the lab without agreeing to and signing the statement below. Please hand it directly to your instructor who will then check off your name on the class list. To ensure that Chem 113/123 labs are carried out safely, I, _________________________ (print name) WILL read and complete the prelab for each lab, and make sure I understand the correct and safe use of equipment and lab techniques. I will comply with all safety instructions given in the lab manual and by the TA and I will report to the TA any and all accidents or injuries. I furthermore agree to look up all hazards of every substance which I will come in contact with for each lab. I also understand that while in the lab I need to wear appropriate eye protection and a cotton lab coat at all times. I will ask questions about any safety issues that I am unsure about. Signed, ______________________________ ________ ___, 201___ Signature ___________________________ Student Number Month Day Year 6 GENERAL REGULATIONS AND PROCEDURES • Regular and punctual attendance of the laboratory sessions is mandatory. • Never work alone in a chemistry laboratory. • Do not attempt or perform unauthorized experiments in the laboratory. • Do not modify any experimental apparatus. • Chemicals and equipment cannot be removed from the laboratory. • Reagents and stock solutions are found on the reagent shelf or reagent bench in the laboratory. A reagent bottle should always be returned to its proper space after use. In order to avoid contaminating reagents, a chemical must never be returned to a reagent bottle. Instead, a sufficient quantity of reagent should be transferred to an appropriate receptacle, e.g. test tube, beaker or watch glass. Excess should be discarded properly, as directed by the instructor or TA. • At the end of the laboratory period, your lab station must be left clean and tidy. All glassware should be thoroughly washed and returned to the drawers, all electrical apparatus should be switched off and unplugged, and all water, gas, and vacuum taps should be fully turned off. 1. Safety Regulations Safety rules will work only if you obey them. Failure to do so will lead to exclusion from the lab. • Never work alone in the laboratory. A laboratory instructor/teaching assistant must be present. • A chemical laboratory is a potentially dangerous environment; the hazards of fire, cuts, burns and poisoning are possible if proper procedures are not used. Follow all directions given by the instructor for the safety of yourself and others. • All accidents and incidents must be reported immediately to the laboratory instructor. • A first-aid kit is provided for the immediate treatment of minor accidents. We have done a risk assessment of all the lab experiments in this manual. Most lab procedures are not dangerous to life and limb. Some, if done incorrectly, may cause harm. A very few lab procedures require a higher level of alertness and safety on everyone’s part. Working with concentrated or hot acids or bases or highly reactive or toxic compounds demand a higher level of safety. During these situations, you will work in a fume-hood and wear safety goggles (not safety glasses). Otherwise, you can wear either safety glasses or goggles. We will indicate when you must wear goggles. Continue to wear your goggles until others directly in front of you and on either side of you are also finished the procedure requiring a higher level of protection. This makes sense. Sometimes it’s not you, but rather a close neighbour who creates a dangerous situation. 2. Personal Safety • NO smoking, eating or drinking in the laboratory. Do not chew gum or apply lip balm. • Protective eyewear (goggles/glasses) must be worn in the laboratory at all times when laboratory work is being carried out anywhere in the room. Your TA will tell you when/if you are allowed to remove your eye protection. Safety glasses and goggles are available for purchase in the UBC-O Bookstore. • The wearing of contact lenses is strictly prohibited in the laboratory. A chemical splashed into an eye quickly moves under the contact lens where it cannot be removed by tears or washing until the lens is taken out of the eye. Irritating substances compound the problem by causing a reflexive closure of the eyelids making the removal of lenses 7 extremely difficult. Even chemical vapours in the room air can cause a problem by dissolving in the film of moisture on the surface of the eye and moving under or even dissolving in the contact lens, causing damage to the eye. For these reasons, prescription glasses must be substituted for contact lenses while working in the laboratory. Students wearing prescription glasses must also wear additional protective eyewear. Safety goggles and certain safety glasses usually fit comfortably over eyeglasses – check before purchase. • If a chemical gets into your eyes, immediately use the eye wash station (know its location) to thoroughly flush the eyes while holding the eyelids open. Call the laboratory instructor. • Students must keep their torso, arms, legs, and feet covered while in the laboratory. No shorts, skirts, low cut blouses, open-toed or open top shoes should be worn. Long hair should be tied back at all times. Improperly dressed students will not be allowed to work in the lab, which may result in a ZERO grade for the experiment. • Students must wear approved, all-cotton lab coats in the Chemistry laboratories. Students should not wear their lab coats outside the Chemistry laboratory. • Many of the chemicals in the laboratory are poisonous whether taken orally or absorbed through the skin. If any chemical is swallowed, the laboratory instructor should be notified immediately. If any chemical comes into contact with the skin it should be washed off immediately with plenty of water and soap. Protective gloves are supplied when needed. IMPORTANT NOTE: Never wear gloves outside the lab. Do not touch your phone, door handles, drawer handles, etc. while wearing gloves as you may contaminate them with chemicals! • When heating a substance in a test tube, ensure that the mouth of the test tube is not pointing at anyone. Never look down a test tube that is being heated. • When heating a liquid, always add boiling chips to the solution. • Concentrated acids and bases, strong oxidizing and reducing agents, flammable solvents and toxic chemicals should be treated with care. • Care should be taken while inserting pipettes, glass rods, etc into rubber, cork or plastic. The correct way is shown here 3. Fire • Students should be aware of the location and use of the fire extinguishers in the laboratory. • In the event of fire, the laboratory instructor must be notified immediately and the flames should be extinguished with one of the extinguishers in the laboratory if it is possible to do so safety. • If a student's clothes or hair catch fire, use the emergency shower or smother the flames immediately with a fire blanket (make sure you know their location). 4. Fumes, Spills, and Breakages • All breakages and minor spills should be reported immediately to the laboratory instructor. • Make sure all broken glass is disposed of in the appropriate container. A receptacle in the laboratory is reserved solely for contaminated glass waste and another one for clean glass waste. • Any experiment involving toxic or corrosive chemicals, producing toxic gases or pungent odours or fumes must be carried out in the fume-hood. • Students are accountable for their own actions in the laboratory and the Chemistry Department will not accept liability for accidents that occur due to irresponsibility on the part students. 8 9 THE LABORATORY NOTEBOOK AND REPORTS Preparing for an Experiment 1. Before entering the laboratory, you must be thoroughly familiar with the details of the experiment as written in the laboratory manual. 2. The “Prelab Assignment,” included in the lab manual at the end of each experiment, must be completed prior to lab each week. The Prelab Assignment sheets will be collected as students enter the laboratory. These questions will be graded as a component of the overall laboratory grade. Students who have not completed the “Prelab Assignment” prior to entering the lab will not be allowed to complete the lab and will receive a grade of ZERO for that lab. 3. Prepare your lab notebook as described below. Laboratory Notebook • The laboratory notebook must be a bound or coil-type notebook to be used to keep a record of all lab work. INK, not pencil, must be used throughout the book. • The first 2 - 3 pages should be left for Table of Contents referring to experiments on pages numbered in ink throughout the book. The pages should be numbered and the Table of Contents set up at the beginning of the semester. • All experimental details and observations are to be recorded directly into this notebook. IT IS NOT TO BE RECOPIED LATER. You may want to use the right hand pages for writing up labs and save the left hand pages for rough calculations. • The notebook does not have to be perfect. Mistakes should be crossed out with a single line. • BEFORE coming to the lab, preparation in your lab note-book (in INK) must include: - TITLE of the experiment and the DATE on which it is to be carried out. - A brief paragraph (Introduction) outlining the PURPOSE of the experiment, any specialized equipment that will be used and containing the chemical reaction equations on which the experiment is based - 1 to 3 sentences giving an outline of the PROCEDURE that will be used. Do not copy the entire procedure from the manual and do not just reference the lab manual. - Prepare a TABLE OF REAGENTS/PRODUCTS for chemicals used in the experiment listing their hazardous properties and any other relevant information. (see MSDS information http://riskmanagement.ubc.ca/health-safety/chemical-safety/materials-safety-data-sheets) There is a designated MSDS computer in Sci 221A for everyone’s use. 1. Prepare tables into which data can be recorded (weights, burette readings, etc.) • 10 IN THE LABORATORY, recorded entries will vary according to the type of experiment but may include: - unknown number and description of unknown samples; - data, e.g. weights, reagent concentrations or titration volumes clearly set out WITH UNITS; - observations and any changes made to the procedure based on the TA’s directions; - calculations Do not erase anything in the lab notebook, including data collected. If data is suspected to be incorrect, cross it out with a single line and note the correction. The original data may sometimes turn out to be correct after all. Scrap paper must not be used for recording data, results and observations. The lab notebook must be used for this purpose. Only once observations have been recorded into the notebook should they be transferred onto the observation sheets. Students recording results on scrap paper may be penalized when the experiment is graded. • The lab notebook will be graded at the end of the term. It is worth 10 marks. It will be graded on usage following the above guidelines. • Note: The lab note book must be initialled by the lab TA during each lab, after your work area is cleaned up and before you are allowed to leave the laboratory. Laboratory Report Two forms of Laboratory Report will be used in this course - informal and formal. For an informal report, you will fill in the calculations and results on the “Report Sheets” supplied in the lab manual. The Report Sheets must be stapled together and submitted for grading. A formal laboratory report gives you a more realistic experience with experimental science. You are responsible for producing a short paper encompassing the chemical basis of the experiment, the experimental methods used, the recorded data, the data interpretation and a conclusion. There is one formal report each term. In upper-level science courses, formal laboratory reports are the norm. The formal reports in first year Chemistry give you an introduction to this format. Format for the Formal Laboratory Report 1. Title Page: A separate page with the following information: experiment title, your name, your partner's name, the instructor's name, course and section number and the date the lab was performed. 2. Introduction: 2 or 3 paragraphs stating briefly what the experiment is designed to accomplish and including balanced equations and a description of specialized equipment where appropriate. 3. Procedure: A brief systematic outline of what was done in the experiment written in the past tense and in the passive voice, (no personal pronouns) ex: "A burette was filled ...” instead of “I filled a burette...”. In particular, any deviations from the given procedure should be noted. Complete sentences must be used. The point of including a procedure is to allow someone to be able to reproduce your experiment. DO NOT copy from the book! Put the steps of the experiment into your own words that someone else could carry out in the same manner to duplicate your results. In science, if you publish a paper and the results are irreproducible, your career may be seriously damaged. 4. Observations and Data: Data should be presented in a table. Identify the units for all data recorded as well as the uncertainty of measurements taken. 5. Calculations: Always show a complete example of the calculations used to interpret the data. Make sure your calculations and data are clear… circle, underline, tabulate, or otherwise list clearly the final answers. You do not want the reader to have to hunt for the answers. Units must be given at each stage of the calculations and, if required, an estimate of the experimental error should be calculated. 11 6. Discussion and Conclusion: This is the most important section of the report. This is your chance to show you understand and can interpret the data. Discuss how theories are proved or disproved by your data and how sources of error affect the results. Things like yield, purity, expected results should also be discussed when appropriate. Summarize your results. Conclusions are drawn from the interpretation of the data and any explanations as to what happened in the experiment. Was the experiment a success? 7. References: This is the last section of a scientific paper. References should be typed in a list at the end of the article and numbered in the order of appearance in the text, tables, or figures. Citations in the text should be denoted by numbers in square brackets as [1]. Web pages are not to be used as primary references. You may use an electronic source if the material is a reproduction of a print article or book, but you should cite the hard copy version using the formats given above. A guide to scientific citation and referencing can be found in the Appendix. Every lab report (informal and formal) has to be signed by the student affirming that the report is the student’s own work. Plagiarism Any form of plagiarism is unacceptable: Copying pre-labs, lab reports, calculations, graphs, tables, etc from another student, including your lab partner is considered plagiarism. Do not read other peoples’ report and do not allow others to read yours. Your personal writing style will be altered easily just by reading someone else’s writing. If you use any source to help you with your lab write up you must reference the source. While the use of quoted passages is acceptable in some circumstances, such as in the critique of a novel, it is very rarely appropriate for a scientific paper or report. You are expected to reword the idea and cite the reference. If you choose to include quoted passages in your lab report or copy a paragraph from some source, you must reference the source, and you should still anticipate a deduction for this lazy practice. Generally, just don’t use quotes in lab reports. The penalty for plagiarism is usually zero on the report but repeated plagiarism may mean more serious penalties. For a detailed discussion on plagiarism refer to the Policies and Regulations/Academic Misconduct/Plagiarism in the UBC-Okanagan Calendar. Evaluation Each laboratory report will be graded on presentation, accuracy, correctness of the data, interpretation of the data, etc. A student working in the laboratory is also continuously being evaluated on experimental technique and general understanding of the experiment. Each pre-lab is worth 2 marks, most informal lab report is worth 10 marks, formal reports are worth 20 marks, the lab notebook is out of 10 marks, and the lab practical exam (at the end of the second term) is worth 25 marks. The final lab mark is worth 20% of the total course mark each term. You must pass both the lecture and lab portions of any Chemistry course independently in order to pass the course. 12 SIGNIFICANT FIGURES When quoting a measurement it is important to know the significance of each number in the expression. The general rule is to quote all places in the number that are known to be certain plus one extra digit. For example, in the figure below, the arrow occurs at 3.58 (approximately). The numbers 3 and 5 are known with certainty, whereas the last number, 8 is somewhat uncertain in its value. The measurement could be quoted as 3.58 + 0.02 where the absolute error is only concerned with the second figure after the decimal point, the one with the uncertainty. To count the number of significant figures in a number, the number is read from left to right and all the digits counted starting with the first non-zero digit. For example: 0.0309 has 3 significant figures 5.3618 has 5 significant figures 500.0 has 4 significant figures 5 × 102 has 1 significant figure 500 gives no indication of certainty The following rules should be applied when carrying out calculations involving experimental quantities. 1. In addition or subtraction, figures in the answer are significant only if each number in the problem contributes a significant figure at the decimal level. Therefore, the value which terminates at the highest decimal level will determine how far the significant figures should be carried in the answer. For example: 308.7812 + 0.00034 + 10.31 should be written 308.78 + 0.00 + 10.31 2. When a number is rounded off (i.e. non-significant figures discarded), the last significant figure is unchanged if the next figure is 4 or less. For example, 83.846 becomes 83.8 when rounded off to 3 significant figures and 0.08999 becomes 0.0900 when rounded off to 3 significant figures. 3. In multiplication and division, the number of significant figures in the answer is the same as that in the quantity with the fewest significant figures. For example, if the volume of a solution was calculated as 2.9268 ± 0.2780 mL and this was obtained by dividing a number with two significant figures (4.8) by a number with three significant figures (1.64), then by the above rule the result should have only two significant figures. In addition, the absolute error has but one significant figure and should be rounded off for the final answer. The volume of the solution is thus 2.9 ± 0.3 mL. 4. In a multistep computation, it is convenient to round off the results of each step so that they contain one significant figure more than necessary. The final answer is then calculated and the number rounded off to the correct number of significant figures. 13 5. When the logarithm of a number is calculated, the number of decimal places in the logarithm must equal the number of significant figures in the original number. For example: log(3.45×10-5) = 4.462 The number 3.45 × 10-5 has 3 significant figures, so the log of 3.45 × 10-5 must have 3 decimal places. To understand this you must remember that log(3.45×10-5) = log(3.45) + log(10-5) = 0.538 – 5 = -4.462 The "-4" in the logarithm (the "characteristic") is determined from the power of 10 in the original number: "-5". The numbers to the right of the decimal point (called the "mantissa") are determined from the number 3.45 which has 3 significant figures. Thus 3 significant figures are required in the decimal part of the logarithm. Similarly when the inverse log of a number is calculated, the number of significant figures in the answer must equal the number of decimal places in the original number. For example: antilog -3.32 = 4.8 × 10-4. In this example, the "-3" is not a significant figure. In effect, it "locates" the decimal point in the answer. Thus only 2 significant figures are allowed in the answer. NOTE: The use of electronic calculators giving answers with 9 or more figures does not alter the above rules regarding significant figures. The number calculated must be rounded off to the correct number of significant figures even if you have to drop many of the figures shown in the calculator display. Calculators do not make your answers any more accurate. ERRORS, UNCERTAINTY, ACCURACY AND PRECISION Most experiments in this course are quantitative in nature; that is physical quantities such as mass, temperature, volume and time are measured. In all measurements a degree of uncertainty is present due to limitations in the instrument or equipment used, the method adopted and the technique of the experimenter (that’s you!). It is important that a quantitative estimate for this uncertainty be obtained. Every measurement has an error, which is the difference between the measured value and the actual or TRUE value. This error is usually not known, because we don’t know the true value. (It is generally very difficult, time consuming, and costly to determine the true value with absolute certainty.) Instead, we report the measured value with an estimated uncertainty. This is a range in which we are confident that the TRUE value resides. For example, the expression 1.02 ± 0.03 grams implies that we are confident the true value lies between 0.99 and 1.05 g. The uncertainties for the glassware and equipment used in your lab have been determined for you and are given in the table on page 16. These values should be used in all uncertainty calculations unless you are otherwise instructed. Note that the terms “uncertainty” and “error” are sometimes used interchangeably (even in this manual), but they are actually different. Uncertainty (and experimental error) arises from two main sources - accuracy and precision. Accuracy is a measure of how closely the value of a physical measurement (or average of a set of measurement) comes to the true or accepted value. Accuracy is sometimes difficult to measure 14 because the TRUE value is usually unknown, but in general it can be estimated by measuring the same physical quantity by another proven technique and comparing the value with the one already obtained. Precision is a measure of the reproducibility of a measurement when carried out several times using the same technique. It is thus a measure of the reliability of both the measuring device and the operator. With care, you will be able to obtain both accurate and precise results in these experiments. Sloppy work, inattention to detail, and/or failure to follow instructions will likely compromise accuracy, precision, safety, and your mark. An example will best show the difference between the two quantities contributing to uncertainty. Consider the following targets obtained from a rifle range: The crosses represent the bullet holes. The targets above can be considered to be analogous to the outcome of a chemical experiment and hence to minimize the experimental uncertainty it is necessary to maximize both accuracy and precision. 15 UNCERTAINTIES The table below shows typical uncertainties associated with apparatus used in this course. Uncertainty in each reading Instrument Electric Top-loading balance Analytical balance 50 mL burette 1 mL pipette 2 mL pipette 3 mL pipette 5 mL pipette 10 mL pipette 15 mL pipette 20 mL pipette 25 mL pipette 50 mL pipette 25 mL volumetric flask 100 mL volumetric flask 250 mL volumetric flask 500 mL volumetric flask 1000 mL volumetric flask pH meter laboratory clock spectrophotometer thermometer 1° calibration 0.1° calibration ± 1 for last figure displayed ± 0.0001 g ± 0.02 mL ± 0.006 mL ± 0.006 mL + 0.01 mL ± 0.02 mL ± 0.02 mL ± 0.03 mL ± 0.03 mL ± 0.03 mL ± 0.05 mL ± 0.03 mL ± 0.1 mL ± 0.1 mL ± 0.2 mL ± 0.3 mL ± 0.05 pH units ±2 seconds ± 0.4% transmittance ± 0.5° ± 0.05° Uncertainty is expressed either in the form of an absolute error or as a percent error (sometimes referred to as relative error). For example: If the mass of a piece of paper on a balance is measured to be 2.5 g, the actual mass can be described as: 16 2.5 ± 0.2 g (absolute error) 2.5 g ± 8% (percent error) or [Note: 8 % = (0.2g/2.5g)*100] Using Uncertainties in Calculations • When adding or subtracting: add absolute uncertainties. • When multiplying or dividing: add their percent uncertainties. Suppose some salt was placed on the above piece of paper and the combined mass was measured to be 7.3 ± 0.2 g then we have: Mass of salt = (7.3 ± 0.2) g - (salt and paper) = (2.5 ± 0.2) g (paper) 4.8 ± 0.4 g Now, if the density of salt was measured to be 1.64 ± 0.02 g/mL, then the volume of the salt can be calculated from the equation: volume = Mass of salt = 4.8 g ± 0.4g = 4.8 g ± 8.3% Density of salt mass density [Note: 8.3 % = (0.4/4.8)*100] = 1.64 g/mL ± 0.02g/mL = 1.64 g/mL ± 1.2% [Note: 1.2 % = (0.02/1.64)*100] Volume of salt = 4.8/1.64 mL ± (8.3% + 1.2%) = 2.927 mL ± 9.5% = 2.927 ± 0.28 mL = 2.9 ± 0.3 mL (Note significant figures) Other Sources of Error: There are a number of errors that appear during an experiment which cannot be assigned a numerical value. These errors may be due to carelessness and/or lack of experience or training. With care and patience these sources of error can be eliminated almost completely. Some common sources of this type of error are listed below: 1. 2. 3. 4. 5. 6. Incorrect reading/recording of a scale Dirty glassware Using a pH (or other) meter that has not warmed up Using a balance that is not level Using a balance that has not been zeroed properly Procedural errors Arithmetical or calculation errors are not considered the same as experimental error because these do not affect the actual data. When the calculation error is discovered, the calculations can be corrected without measuring more samples. If one of the errors listed above is discovered, the measurement(s) must be discarded and redone. 17 GENERAL LABORATORY TECHNIQUES Volumetric Flask Volumetric flasks are used to make up solutions of accurately known concentrations. To dissolve a solid in a volumetric flask, the desired amount of solid is weighed out on an analytical balance and transferred to a volumetric flask. The weighing container is rinsed with distilled water several times and the rinsings are added to the flask. This is called quantitative transfer. The volumetric flask is partially filled with distilled water until the level is several centimetres below the fill line. The flask should be swirled frequently to dissolve the solid (this may take several minutes).When the solid has completely dissolved, add distilled water to the flask until the bottom of the meniscus is at the mark on the neck of the flask. Use a pasteur pipette to add water dropwise near the end. Cap the flask securely and invert it fully. When the neck is completely full of solution, right the flask and allow the air to return to the neck. Repeat this at least 10 times to insure complete mixing of the solution. To dilute a solution, use a pipette to transfer an accurately known volume of a concentrated solution to a volumetric flask. Add distilled water to the flask until the bottom of the meniscus is at the mark on the neck of the flask. Cap the flask and invert it at least 10 times as above to insure complete mixing of the solution. INCOMPLETE MIXING WILL ALWAYS GIVE POOR RESULTS. The Pipette The pipette is used in the laboratory to deliver accurate volumes of solution. Pipettes are of two kinds: (i) those which have one calibration mark and deliver a constant volume of liquid (transfer pipettes), and (ii) those in which the stems are graduated and can be employed to deliver various volumes of liquid (graduated pipettes). NOTE: If the pipette is not clean and dry, prior to use, it should be rinsed 2-3 times with ~1 mL of the solution to be pipetted. Do not completely fill the pipette to rinse it – just tip it back and forth. The pipette is filled by immersing the tip deep into the solution and applying suction on the other end with a partially deflated rubber bulb until the pipette is filled to a level slightly above the calibration mark. The rubber bulb is removed and the index finger is quickly slipped over the upper end of the pipette to hold the solution in place. Easing pressure on the end of the pipette, the solution is allowed to run out until the bottom of the meniscus is level with the calibration mark. The pipette now contains slightly more than the specified volume; the pipette is calibrated to deliver (TD) the specified volume when allowed to empty naturally. After delivering the specified volume, some liquid will remain in the tip of the pipette; this should not be removed by blowing down the pipette. To ensure that the amount specified is delivered in full, the tip of the pipette should be tilted and held against the side of the container. NOTE: Avoid sucking liquid into the rubber pipet bulb. If any liquid accidentally enters the rubber bulb, it should be thoroughly rinsed out with distilled water and set aside to dry. The Burette The burette is used to deliver measured quantities of liquid to a container. The volume added is determined by the needs of the experiment and is quite variable. Burettes are generally of 50.00 mL graduated capacity, the graduations being at 0.1 mL. The burette reading can be estimated to ± 0.01 mL. Burettes with other volumes and graduations are available. 18 NOTE: Prior to use, the burette should be rinsed carefully with a few mL of the solution to be used for filling. The burette is filled by pouring liquid into the top with the stopcock closed; the stopcock is then opened and the stopcock and delivery stem below the stopcock filled with liquid (ensuring that there are no air bubbles). This latter operation is extremely important. Liquid is added to a container from the burette through the stopcock. The exact amount of liquid transferred can be calculated by taking the difference between the burette reading before and after the addition. When reading a burette, the scale reading at the bottom of the meniscus is taken (see diagram below). NOTE: The burette must be mounted vertically (straight up and down) to give correct readings. Titration Titration is a technique used in volumetric analysis to determine the concentration of a substance in a solution. Titrations involve determining the volume of a solution of accurately known concentration (the standard solution or titrant) that is required to react completely with a solution of the substance being analyzed (the analyte). The weight or concentration of the substance to be determined is then calculated from the volume and known concentration of the added standard solution and the stoichiometry of the reaction. An accurately known volume (e.g. 5.00 mL dispensed from a volumetric pipette) of a solution to be analyzed or a known weight of solid sample is placed in an Erlenmeyer flask and the standard solution is added from a burette. The point at which a sufficient volume of the standard solution has been added to react completely with the sample is termed the end-point and is detected by use of a pH meter or an indicator which changes colour at the end-point. • Care is needed when approaching the end-point in a titration. Once momentary colour change appears, add only drops of the standard solution until the end-point is reached. • A piece of white paper placed under the Erlenmeyer flask makes it easier to see the colour change at the end point. • A white card makes it easier to read the meniscus of the burette. Filtration Filtration is the process used to separate a solid from a liquid. Both solid and liquid are poured onto a filter which is impermeable to the solid and permeable to the liquid. The filter is generally made of paper or sintered glass. 19 The gravity filtration process uses a glass short stem funnel and large-diameter filter paper folded into a cone. Some general points to note for this process are as follows: 1. The filter paper is prepared for insertion into a short stem funnel by folding it in half along one diameter of the filter paper, then in quarter by a further fold, as in the diagram. 2. For rapid filtration, the filter should fit snugly in the funnel so that air cannot be drawn down between the paper and the funnel. 3. The paper is inserted in the funnel and moistened with water from a wash bottle, then pressed gently against the funnel wall. When the funnel is properly sealed, liquid poured into the funnel, will be retained in the filter stem. If liquid is not retained in the filter stem, either the paper is not sealed or the stem is dirty, in which case it should be cleaned. 4. When pouring a mixture into a filter, it should be poured down a stirring rod. A wash bottle should be used to wash any residue adhering to the walls of the container into the filter. 5. The liquid level in the filter funnel should not rise more than 2/3 up the filter paper. Vacuum filtration process uses a Buchner funnel and a smaller diameter filter paper. A vacuum is applied to speed up the filtration process. Some general points to note for this process are as follows: 1. A clamp must be used to secure a side-arm flask to a retort stand. The flask is then attached to a vacuum line. 2. A rubber adapter (often called a FilterVac®) is placed on top of the flask with the flat side facing down. The adapter must be centered to ensure a good seal. A Buchner funnel is then placed in the centre of the FilterVac. 3. Filter paper of the appropriate size is inserted in the Buchner funnel. The paper should cover all holes but not extend up the walls of the funnel. Paper can be cut to fit if needed. 4. The vacuum is turned on and filter paper moistened with water from a wash bottle. It may be necessary to push down on the funnel to seal the system. 5. When pouring the mixture into the filter, it should be poured slowly down a stirring rod into the centre of the paper. Pouring too fast can cause the filter paper to lift and mixture to seep underneath it. Solid and liquid will then not be separated. 20 INSTRUMENTS Balances The use of the various types of balances that are available in Chemistry laboratories will be demonstrated in the introductory lab sessions. Balances are fundamental to Chemistry laboratory experimentation and you must become adept in using them. The two types of balances are toploading and analytical. Note that we do not call them “scales!” For the weighing of approximate quantities of materials, a top-loading balance is suitable. For accurate weighing, the analytical balance must be used. Top-Loading Balance: Most of our top-loading balances can measure mass to ±0.01g and a few are capable of ±0.001g. These balances are generally used when the precision of the measurement is not critical, or when the mass is very large (<100g). A common use is measuring out a reagent for a synthetic chemical reaction. In this case, stoichiometry usually requires that the amount of reagent be close to the recommended amount such that the 2 decimal places are adequate. Analytical Balance: Analytical balances have higher precision than top-loading balances and are able to measure mass to ±0.0001g (0.1 mg). This high precision is usually only required for chemical analysis – procedures designed to accurately determine the amount of a certain chemical species. Occasionally, when masses < 100mg (0.1 g) are required, then a toploading balance is generally not sensitive enough so an analytical balance should be used. A trade-off for the higher precision is that these balances are not as robust as the top-loading balances and care must be taken when using these expensive, delicate instruments. General rules for using balances: 1. Always check the balance is level and at zero prior to a weighing. 2. Never weigh a chemical directly on the balance pan. Always use a receptacle such as a beaker, weighing bottle or weighing paper. 3. Do not weigh hot objects on a balance – let them cool first. 4. Any spillages on or near the balances should be immediately cleaned up with the provided balance brushes into the waste beaker. 5. When estimating the experimental uncertainty of a weighing, remember there is an uncertainty in the zeroing (taring) of the balance as well as in the measurements. 6. In an experiment, the statement "accurately weigh out about 1 g of compound X" may be used. This means that, using an analytical balance, about 1 g of X should be weighed out and that this weight should be known accurately. The actual weight can be between 0.9500 g and 1.0500 g. The statement means that you should not waste time trying to weigh out exactly 1.0000 g of X. 21 The pH Meter The pH meter is a meter used to measure the pH of aqueous solutions. solutions It consists of two electrodes which may or ma may y not be combined into a single housing. The electrodes are immersed in the solution under investigation. investigation One of the electrodes is a reference; the other is sensitive to the presence of the acid species H+ (or H3O+). When the electrodes are immersed in a solution, olution, a cell is set up which has a potential difference (voltage) that is related to the solution pH pH. This cell potential is measured by the instrument and converted directly to give a pH value on the meter scale. Operation 1. Take the electrode out of the storage solution, rinse with water from a wash bottle, and dry by gently dabbing with a Kimwipe®. 2. The electrode must be calibrated prior to use. a. Press the CAL button on the pH meter to begin calibration. b. Insert the electrode into the pH 4 buffer. Pres Press ENTER. Rinse and dry the electrode. c. Repeat with a pH 7 and pH 10 buffer, rinsing and drying the electrode between solutions. 3. Once the calibration is complete, the display will read MEAS. The electrode may be stored in the storage solution until needed. Do not turn the meter off or the calibration will have to be redone. 4. To conduct measurements, rinse and dry the electrode, then immerse it in the solution of interest. The display will show the pH of the solution The calibration of the meter and the actu actual al pH measurement will be demonstrated by the Lab Instructor. The instruction manual is also available. Notes 1. Rinse the electrode with water from a wash bottle and gently pat dry with a Kimwipe® each time you change solutions to prevent contamination. 2. The electrodes are fragile and should be treated with care care. Particular care must be exercised when washing the electrodes as a scratch on the surface may ruin it and necessitate the costly replacement of the electrode. They are expensive expensive, so please be careful! 3. The buffer solution should be used to recalibrate the meter from time to time.. The pH of the buffer solution will remain constant and need not be discarded after each calibration. calibration A buffer solution should be used that has a pH close to that of the solu solution tion being investigated. The Spectrophotometer The spectrophotometer is an instrument that measures the absorbance or % transmittance of a solution at different wavelengths of incident light. If a beam of light of a specific wavelength is made to pass through a liquid solution solution, some of the light may be absorbed by the solution, and the rest is transmitted transmitted. The transmittance (and percent cent transmittance) transmittance is defined by the equation: 22 transmittance = intensity of transmitte d light intensity of incident light % transmittance = transmittance × 100% In an optically transparent solution, all the light at the specific wavelength passes through the sample and hence the % transmittance is 100%. If some of the light is absorbed then the % transmittance will be less than 100%. The absorbance of the solution is defined: absorbance = − log10 ( transmittance) The absorbance of a solution is directly proportional to the concentration of the absorbing species in the solution Spectronic 20D+ Operation 1. Turn on using lower left-hand knob (0%T knob) – allow instrument to warm up for 15 min. 2. Select wavelength using upper knob – set filter lever on lower left to appropriate range based on the wavelength selected. 3. Set 0%Transmittance: Push ‘Mode’ button until ‘Transmittance’ is selected (small red light on display). With sample compartment empty, rotate lower left-hand knob to get a reading of zero. 4. Set 0 Absorbance: Push ‘Mode’ button until ‘Absorbance’ is selected. Place a cuvette filled with an appropriate reference solution in the sample compartment. Rotate lower right-hand knob to get a reading of zero. 5. Replace the reference cuvette with the sample cuvette and record the reading. 23 GRAPHING A well designed graph of experimental data is a very effective device for observing trends, discovering relationships, or predicting information. A graph is constructed on a set of perpendicular axes. The vertical axis (the Y axis) is the ordinate, and the horizontal axis (the X axis) is the abscissa. 1. The independent variable (the variable that is controlled in the experiment) is plotted along the horizontal X-axis, with values of the dependent variable (the variable that responds to change) along the vertical Y-axis. For example, if the experiment was conducted by varying the pressure and measuring the resulting volume, the independent or controlled variable - pressure - would be plotted on the X-axis and volume on the Y-axis. This is a plot of Volume vs. Pressure (Y vs. X). Always label the graph, the axes and indicate the units. 2. Choose scales for the X and Y axes that cover the ranges of the experimental data. This should allow the data to cover as much of the space on the graph as possible. For example: If experimental data ranged from 317 to 747 torr, choose a pressure scale that ranges from 300 to 800 torr. This covers the entire data range and allows us to mark the major divisions at intervals of 100 torr. When dividing the scale, always choose values for the major divisions so the smaller subdivisions can be easily interpreted. For example: Major divisions every 100 torr will easily allow subdivisions every 10 torr. Therefore plotting data values such as 475 torr will not be difficult. Also: - values do not have to begin at zero at the origin. - the scale should reflect the same accuracy to which the data was taken. - the range of the scale should cover any range of extrapolation you will make. 3. The scale for the Y-axis is created using the same techniques. 4. Consult the Excel demo in Lab A for more details. 24 6. Every graph requires a descriptive title giving clear and specific information about the graph. Describe both variables (e.g. “Distance versus Time” NOT “m vs s”) and include the purpose for which the graph is drawn (e.g. “To determine Rate”). Do not just state the "Y" variable versus the "X" variable as a title. Place a descriptive title in an area away from the data points and the line. Under the title include your name and date. Graphs should be labelled in ink with data points and graph lines in a sharp pencil. For Straight Line Graphs Some relationships produce a straight line. This is the case when we plot pressure versus temperature for a fixed volume. Excel has a function which draws a line corresponding to the best fit of data points. A straight line is described by the equation: y = mx + b where m is the slope and b is the point of intersection of the line with the y-axis when x = 0 as shown in the graphs below. The slope of the line, which usually is of greatest interest, is calculated as: m= y2 − y1 ∆y = x 2 − x1 ∆x 25 26 Lab A. USING EXCEL FOR GRAPHING This lab is an exercise to assure that you know how to prepare acceptable graphs of scientific data in Excel. Excel can be used as a tool for preparing various types of graphs. In this lab, you will learn how to draw the simple graphs that you will need to pass the first year chemistry lab. EXCEL SPREADSHEET • • Click on Excel icon. A new spreadsheet will appear. Enter the following hypothetical experimental data in the spreadsheet: Concentration (mg/L) 0 1 2 3 4 5 7 6 8 9 10 Absorbance 0.006112 0.029012 0.069122 0.078256 0.106808 0.144596 0.186333 0.155391 0.202737 0.234936 0.279798 This experiment shows the variation of the absorbance with concentration. • Highlight the data. • Click the Chart Wizard button on the toolbar. Select: XY (Scattered) for Chart Type and click the Next button. DO NOT use a line chart – it is not the same as XY scatter. • Click the Next button again. • Under Titles add the title: “Absorbance versus Concentration” for the chart, for the X-axis type in: “Concentration (M)” and for the Y-axis type in: “Absorbance”. • Under Gridlines, remove unwanted gridlines by clicking away the appropriate check boxes. • Under Legend, remove the legend by clicking away the check mark in the Show Legend box. Click the Next button. • Leave the chart as an object in the current sheet. You may also select the As new sheet option if you want a bigger graph. Click the Finish button. • Your graph appears in a box. It is already in the Edit mode. Try clicking outside the box and see what happens. Click anywhere within the chart box to put the chart back into the Edit mode. • From the Chart menu at the top of the screen, select Add Trendline. In the Add Trendline dialog box, select the Type tab and then choose the Trend/Regression type that matches your mathematical model. For work in first year chemistry, this will always be a linear fit. 27 • • • • • Use another type of fit only if you have a good reason – that is you have a mathematical model for your data that requires the non-linear fit. Click the Options tab and under the Trendline name select the Automatic option button. Finally select Display equation on chart check box. Click the OK button. Click anywhere within the chart box to remove unwanted details from the graph. You can also move the equation around, by clicking on it and dragging the equation box to another position. Now you can use the displayed equation to estimate the concentration of a sample from its absorbance. For example: if Absorbance (Y) = 0.2, then Concentration X = (Y – 0.00584)/0.02598 = 7.47 mg/L. Absorbance vs. Concentration 0.3 Absorbance 0.25 0.2 0.15 y = 0.02598x + 0.00584 R² = 0.99148 0.1 0.05 0 0 2 4 6 Concentration (mg/L) 28 8 10 12 Lab B. THE DETERMINATION OF DENSITY AND THE APPLICATION OF UNCERTAINTIES Introduction In this experiment the density of a liquid and of a solid will be determined. The density of a substance is a measure of the concentration of mass within the volume of the substance, as defined by the equation: density = mass/volume. The density of the liquid will be determined by using different pieces of volumetric glassware to measure out a known volume of the liquid and then determine the mass using an analytical balance. The density of a solid will then be determined by weighing the solid, followed by measuring the volume of liquid the solid displaces. As well as introducing the student to the important laboratory techniques of weighing and using volumetric glassware, the experiment is designed to familiarize you with the fundamentals of uncertainties and error analysis. Every measurement has an error, which is the difference between the measured value and the actual or TRUE value. This error is usually not known, because we don’t know the true value. (It is generally very difficult to determine the true value with absolute certainty.) Instead, we report the measured value with an estimated uncertainty. This is a range in which we are confident that the TRUE value resides. For example, the expression 1.02 ± 0.03 grams implies that we are confident the true value lies between 0.99 and 1.05 g. The uncertainties for the glassware and equipment used in your lab have been determined for you and are given in the table on page 16. The correct method of using and reading of the equipment to be used will be demonstrated by your instructor. Read the introduction sections of the lab manual for additional information on error analysis and precision (page 14), and use of equipment (page 21). Safety This experiment requires safety glasses during the entire lab period! Procedure The experimental data should be recorded in your lab notebook. You can then transfer the data to the Observations/Report sheet. Note that the report sheets are designed to show you how tables should be prepared, including proper organization, headings and units. You will find that headings and units are provided for you in early laboratories, and you will need to enter them yourself as you become more experienced. Part A. Burette Readings Several sealed burettes are set up for practicing burette readings. Burettes should be read to the nearest 0.05 mL. Reading the burette is described on page 19. Part B. Liquid Density 1. Using an analytical balance, weigh the clean, dry weighing container provided by your TA. 2. Using a pipette bulb and a 10 mL pipette, pipette into this container 10.00 mL of the unknown liquid and cover it. 3. Re-weigh the covered weighing container and liquid. 4. Calculate the density of the liquid and the experimental uncertainty in the density. 29 Part C. Liquid Density 1. Using an analytical balance, weigh a clean dry weighing container provided by your TA. 2. Using a 50 mL burette, add 8 to 9 mL of the unknown liquid, measured accurately, to the weighing container and cover it. 3. Reweigh the weigh container and liquid. 4. Calculate the density of the liquid and the experimental uncertainty. Part D. Liquid Density 1. Using an analytical balance, weigh a dry 25 mL volumetric flask with stopper or cap. 2. Using a beaker and Pasteur pipette, fill the flask to the graduation line with the unknown liquid and stopper the flask. 3. Reweigh the flask on the analytical balance. 4. Calculate the density of the liquid and the experimental uncertainty. Part E. Liquid Density 1. Obtain a 50 mL Erlenmeyer flask (clean and dry) and cork and weigh on an analytical balance. 2. Pipette 15.00 mL of the unknown solution into the Erlenmeyer flask and reweigh. 3. Without emptying the flask, pipette another 15.00 mL and reweigh. 4. Repeat step #3 two more times. 5. Calculate the density for each addition and the experimental uncertainties. Part F. Solid Density 1. Add about 4 to 5 mL of water to a 10 mL graduated cylinder and record the volume to the nearest 0.1 mL (use ±0.1 mL for the uncertainty of the graduated cylinder). 2. Select a metal sample and record its letter. Weigh this sample on a top-loading balance. 3. Carefully slide the metal sample into the graduated cylinder, dislodge any air bubbles, and record the new liquid level. The difference of the readings corresponds to the volume of the metal. 4. Calculate the density of the solid and its experimental uncertainty. Part G. Solid Density 1. Using the same metal sample that was used in part F, dry the metal and reweigh using an analytical balance. 2. Half fill a 50 mL burette with water and read and record the volume. 3. Now slide the metal sample down the burette into the water. Important – Be careful!! Do not drop the metal sample into the burette – this may break the burette $$). Instead, hold the burette at a shallow angle (<45o) and slide the metal down. 4. Tap the burette gently with your finger to remove air bubbles that may be trapped around the metal. 5. Read the burette again and record the volume. 6. Calculate the density of the solid and its experimental uncertainty. Note: Uncertainties for the equipment used in this experiment should be obtained from the table on page 21 of this manual. Waste Dispose of unknown liquids in the provided waste beakers. 30 Laboratory B Determination of Density and Application of Uncertainties PRELAB ASSIGNMENT Page 1 of 1 Student Name: _____________________ Date: ____________ Course: _______ Section: ____ Exercises on Uncertainties and Significant Figures 1. 2. How many significant figures do the following numbers contain: 47.3096 ______ 6.983 x 102 _____ 60.0 __________ .0000530 _____ 30.001 _________ 6.30 x 10-3 _____ From the table on Intro-17 of your lab manual, correctly write down the precise volumes and uncertainties of the following pieces of glassware: (The first one has been done for you). Glassware 3. Absolute Volume Uncertainty Relative Volume Uncertainty 500 mL Volumetric flask 500.0 ± 0.2 mL 500.0 mL ± 0.04% 25 mL Pipette ________________ ________________ 250 mL Volumetric flask ________________ ________________ 1 mL Pipette ________________ ________________ Do the following conversions: (show calculations with all units) 758 cm x (_____ m cm ) = ________m 1.37 mg x (______) = ________g 4. 1500. _____ 9.31 x 105 g x (_____) = ___________kg 0.0146 L x (__________) = _________mL If 110. g of ethanol is needed for a chemical reaction how many litres of ethanol would be required? (The density of ethanol is 0.789 g/mL). The density of a liquid is equal to its mass divided by its volume [d = mass (g)/volume (mL)]. Give your answer in scientific notation with the correct number of significant figures. Show your work. 31 32 Laboratory B Determination of Density and Application of Uncertainties REPORT SHEET Page 1 of 4 Student Name: _____________________ Date: ____________ Course: _______ Section: ____ Part A Buret Readings Run # Volume (mL) Absolute Error (mL) 1 ± 2 ± Part B Item Mass (g) Absolute Error (g) bottle + lid % Relative Error ± (measured weight) bottle + lid + liquid ± (measured weight) Liquid ± ± (calculated weight) Volume of liquid ______________±_________ mL (±_______%) Density of liquid _____________ g/mL±_______ % ______________±__________ g/mL Part C Item Mass (g) Absolute Error (g) % Relative Error bottle + lid ± bottle + lid + liquid ± liquid ± ± Absolute Error (mL) % Relative Error Burette Reading Volume (mL) Initial ± Final ± Volume ± Density of Liquid ± ______________ g/mL ±_______ % _______________±__________g/mL 33 Laboratory B Determination of Density and Application of Uncertainties REPORT SHEET Page 2 of 4 Part D Item Mass (g) Absolute Error (g) bottle + lid ± bottle + lid + liquid ± liquid ± % Relative Error ± Volume of liquid ______________±_________ mL (±_______%) Density of liquid _____________ g/mL±_______ % ______________±__________ g/mL Part E Item Mass (g) Absolute Error (g) flask + cork ± flask + cork + 15mL ± 1st 15mL aliquot ± flask + cork + 30mL ± 2nd 15mL aliquot ± flask + cork + 45mL ± 3rd 15 mL aliquot ± Volume of liquid in each aliquot Item ± ± ______________± ___________mL(± % Relative Error 1 15mL aliquot ± ± 2nd 15mL aliquot ± ± 3rd 15 mL aliquot ± ± Average density of liquid 34 ± Absolute Error (g/mL) st Density (g/mL) % Relative Error ______________±__________g/mL %) Laboratory B Determination of Density and Application of Uncertainties REPORT SHEET Page 3 of 4 Student Name: _____________________ Date: ____________ Course: _______ Section: ____ Part F Unknown Letter ___________ Item Mass (g) Mass of Unknown Item Absolute Error (g) ± Volume (mL) % Relative error ± Absolute Error (mL) Volume of liquid ± Second volume reading ± Volume of Unknown ± Density of Unknown ± % Relative error ± (Reminder: always include units!) Part G Unknown Letter ___________ Item Mass (g) Absolute Error (g) % Relative error Volume (mL) Absolute Error (mL) % Relative error Mass of Unknown Item Initial buret reading ± Final buret reading ± Volume of Unknown ± Density of Unknown ± ± (Include units!) (Continued on next page) 35 Laboratory B Determination of Density and Application of Uncertainties REPORT SHEET Page 4 of 4 Questions: In the following questions, absolute uncertainties for mass, volume, and density must be included (Refer to page 16 in the Lab Manual) and your answers are to be rounded off and reported to the correct number of significant figures. SHOW CORRECT UNITS FOR EACH VALUE. 1. A student uses one of our 10 mL pipettes to weigh out a known volume of liquid into a small beaker. Using the top loading balance, the beaker weighed 9.2 g and 19.4 g when empty and filled, respectively. a) What is the mass of the liquid? ___________________±____________ b) What is the volume of the liquid? ___________________±____________ c) What is the density of the liquid? ___________________±____________ 2. A second student uses a 25 mL volumetric flask and the analytical balance to determine the density. The mass of the flask is 85.3729 g and 110.8733 g when empty and filled, respectively. a) What is the mass of the liquid? ___________________±____________ b) What is the volume of the liquid? ___________________±____________ c) What is the density of the liquid? ___________________±____________ 3. A third student weighs out a volume of the liquid using a 50 mL buret and two different balances. The empty beaker weighed 9.2 g on the top loading balance and the filled beaker weighed 33.5213 g on the analytical balance. The initial burette volume was 0.52 mL while the final volume was 24.54 mL. a) What is the mass of the liquid? ___________________±____________ b) What is the volume of the liquid? ___________________±____________ c) What is the density of the liquid? ___________________±____________ 4. Calculate the average (including error) of: Student Signature: 36 21.5 ± 0.2 g and 22.7 ± 0.8 g? (Show your work) Lab C. WATER OF HYDRATION: DETERMINATION OF AN EMPIRICAL FORMULA Introduction Water is chemically bonded to many substances because of its polar nature and electronic structure. One class of compounds where this bonding with water is prevalent is inorganic salts. When aqueous solutions of salts are evaporated, the salt crystals that remain often contain the salt and water combined in definite proportions. The anhydrous salt has been converted by the process into a hydrate. Common examples of inorganic salt hydrates are Copper(II) sulfate pentahydrate CuSO4•5H2O Magnesium sulfate heptahydrate MgSO4•7H2O Cobalt(II) chloride hexahydrate CoCl2•6H2O Barium hydroxide octahydrate Ba(OH)2•8H2O This chemically bonded water, also known as water of crystallization, is held in the crystal more or less firmly. Some hydrates like Na2SO4•10H2O lose some of their water molecules when exposed to air. Such compounds are said to be efflorescent. The empirical formula of an efflorescent substance is variable when the substance is exposed to air. Other molecules such as anhydrous MgSO4 pick up moisture from the air to form hydrates. Such compounds are said to be hygroscopic. Hygroscopic compounds are useful as drying agents or desiccants. Calcium chloride is an extremely hygroscopic salt that can absorb so much moisture from the air that it dissolves itself, forming a liquid. Compounds showing this behaviour are called deliquescent. Many hydrates are neither hygroscopic nor efflorescent but form hydrates with a definite proportion of salt to water. However, these hydrates can become hygroscopic at elevated temperatures when one or more water molecules are driven off. For example, the substance Plaster of Paris is made from the mineral gypsum by heating the latter to a temperature above 128°C. At a temperature of 163°C or more the substance anhydrite is formed. 2(CaSO4•2H2O) Gypsum CaSO4•2H2O Gypsum 128o C → 163o C → 2CaSO4•H2O + 2 H2O Plaster of Paris CaSO4 + 2 H2O Anhydrite Experimental Method In the first part of this experiment we will heat two coloured hydrates and note a change in colour and the evolution of steam. The dehydrated salt will then be re-hydrated and the reverse process will be observed. In the second part of this experiment you will obtain a salt with an unknown number of waters of crystallization (example Na2SO4•X H2O). The object will be to determine the salt to water ratio for this hydrate. A particular salt may exhibit more than one ratio. The value of X may vary from 0 for anhydrous compounds to 12 for hydrates. You will weigh the hydrate, drive off the water with heat and reweigh the now anhydrous sample. The loss of mass is due to the water. The mass ratio of anhydrous salt to water must be converted to a mole ratio which is then reduced to small whole numbers, called the empirical formula. 37 Safety Flame is used in this experiment. Safety glasses are required during the entire lab period! Long hair has to be tied back. Do not point any test tube towards yourself or your neighbour. Do not touch hot crucibles to avoid serious burns. Use crucible tongs. Procedure Note: To complete this experiment in one laboratory period start with Part B, working on Part A during the cooling periods. Part A. Testing of Hydrates Figure 1 Dehydration of Hydrates 1. Place a spatula tip full (no more than the size of a small pea) of copper (II) sulfate pentahydrate in a large test tube and assemble the apparatus as shown in Figure 1. Carefully follow the instructions given for lighting the Bunsen burner. Be aware that the flame can be hard to see. NEVER leave the flame unattended. 2. Gently heat the base of the tube and note the appearance of water at the mouth of the tube. A change in colour will accompany the dehydration. Record your observations. When the colour no longer changes, allow the tube to cool. 3. Once the tube is cool, add a few drops of water. Stir the sample. Record your observations. 4. Repeat this procedure with a similar amount of cobalt (II) chloride hexahydrate. Record your observations. Part B. The Formula of a Hydrate 1. Heat a clean crucible with a lid for 5 min (10 min. if wet) in the flame of a Bunsen burner to drive off any moisture, as shown in Figure 2. From this point on handle the crucible and cover with tongs only. Serious burns may result from touching hot crucibles. In addition, touching the crucible may change its weight and lead to inaccurate results. Allow the crucible and lid to cool before transporting them on a watch glass or in a small crystallizing dish to the weigh room. 2. After cooling, weigh the empty crucible and lid on an analytical balance. Place about 3 g of an unknown hydrate in the crucible and reweigh the crucible, lid and contents on an analytical balance. Record the weight of the unknown hydrate accurately to 4 decimal places. 3. Again position the crucible as shown in Figure 2. Heat the crucible gently for 5 minutes (with the lid on to avoid spattering) and then strongly for about 15 minutes (with the lid partly off). Cover the crucible, allow it to cool, and then weigh it with the cover on the analytical balance. 38 4. Reheat the crucible strongly for another 5 minutes, allow it to cool and reweigh as before. The two weighings should agree within ±0.003 g. If not, repeat the heating, cooling and weighing as before until repeated weighings are within ±0.003 g of each other. If time allows, repeat this procedure with a second sample of the same unknown. Figure 2 Heating a Crucible Waste Dispose of all solids in the solid waste. Scrape solids out of the crucible. Rinse the crucibles with water from a wash bottle into the supplied waste beaker. 39 40 Laboratory C Water of Hydration PRELAB ASSIGNMENT Page 1 of 1 Student Name: ______________________ Date: ________ Course: ______ Section: _____ 1. Define anhydrous: 2. Define hygroscopic: 3. Define efflorescent: 4. What is the empirical formula of manganese(II) sulfate tetrahydrate? 41 42 Laboratory C Water of Hydration REPORT SHEET Page 1 of 3 Student Name: _________________Date: __________Course: _____Section: ______ Data: Part A. Testing of Hydrates - Record your observations in the space provided. CuSO45H2O CoCl26H2O Is moisture evolved? Describe any colour or other change on heating. Is residue water soluble? Describe any colour or other change on the addition of water. Part B. The Formula of a Hydrate Unknown sample number __________ . Item Formula of Anhydrous Salt ______________________ (This will be provided by your instructor). Mass Run 1 (g) Mass Run 2 (g) Mass Run 1 (g) Mass Run 2 (g) Crucible, Cover and Unknown Crucible and Cover Unknown After Heating Crucible, Cover and Unknown After first heating After second heating After third heating (if required) Anhydrous Unknown (circle driest mass above used in calculations) Mass of Water Lost 43 Laboratory C Water of Hydration REPORT SHEET Page 2 of 3 Calculations: Complete the table by calculating the following from Part B. Item Mass of anhydrous salt Run 1 Run 2 Molar mass of anhydrous salt Moles of anhydrous salt Mass of water evolved Molar mass of water Moles of water evolved Simplest whole number ratio of water to salt (i.e. X:1) Average whole number ratio of water to salt Write the empirical formula of the hydrate._______________________________ Name the empirical formula.__________________________________________ 44 Units Laboratory C Water of Hydration REPORT SHEET Page 3 of 3 Student Name: _________________Date: __________Course: _____Section: ______ Questions: 1. If the hydrate is not completely dehydrated, would the experimentally determined molar ratio of salt to water change? Explain your answer. 2. Is the dehydration of CuSO4.5H2O a physical or chemical change? Explain your answer. 3. Does water of hydration bond to the inorganic ions again when the salt is dissolved in water? Explain your reasoning based upon your observation of copper (II) sulphate. Student Signature: 45 46 Lab E. SPECTROPHOTOMETRIC DETERMINATION OF IRON Introduction In our diet, iron is regarded as an essential trace metal. A healthy human adult contains between 2 g to 5 g of iron; the high end of this range applies to pregnant women. Of the 15 mg or so of iron that should be in our daily diet (red meat and cereals) about 2 mg is absorbed through the intestinal wall. About 65% of the iron in the body is present in the molecules, hemoglobin and myoglobin, responsible for oxygen transport. A very small amount of iron is present in some enzymes, e.g. catalase, and the bulk of the remainder is stored as ferritin or hemosiderin in the bone marrow. The daily turnover of iron through metabolic activity is about 20 mg, of which some is excreted and the remainder recycled. Deficiencies in iron can come from lack of iron in the diet but may also be due to vitamin B12 or copper deficiency and also other metabolic or genetic disorders e.g. sickle-cell anaemia. Iron deficiency, or anaemia is thought to occur in about 12% of North American women and may be as high as 40% in pregnant women. This is primarily due to dietary deficiency (not enough liver lovers!). Chronic anaemia may be alleviated by injections, but a small iron deficiency can be overcome by taking iron tablets as a dietary supplement. Iron tablets normally contain iron complexed with a sugar, e.g. iron fructose. The sugar helps to maintain the iron in a soluble state and to aid in its transfer across the intestinal wall. There are several different methods for the determination of iron. In this experiment you will use a spectrophotometric method for analysis. Spectrophotometric Analysis When a molecule absorbs radiant energy in the visible region of the electromagnetic spectrum, valence or bonding electrons are raised to higher energy orbitals. The amount of energy absorbed by the sample at any particular absorbing wavelength varies directly with the concentration of the sample. Thus, a plot of absorbance intensity as a function of concentration of the sample (Figure 1) will give a straight line with the origin at zero. Standardization Graph for Fe2+. Absorbance as a function of concentration (ppm). To be used to determine [Fe2+] in an unknown 47 The relationship of absorbance intensity to concentration comes directly from Beer's Law. A = abC where A = absorbance (unitless) a = molar absorbance coefficient, a molecular property which is more or less independent of concentration but does vary with wavelength. b = thickness of sample C = concentration of sample If one keeps the wavelength and sample thickness constant then a and b remain constant and A = (constant) C, or A α C (The absorbance is directly proportional to the concentration.) The latter equation is the basis for determining the concentration of an unknown sample of iron by measuring its absorbance A and determining the sample concentration from a plot of A vs. C taken at the same wavelength. The A vs. C plot is obtained by measuring A for samples of known C value. What wavelength does one use? The best wavelength to use is the one which gives the maximum absorbance for a particular sample. To determine this wavelength one can obtain the spectrum of the sample by measuring the absorbance of a solution over a range of wavelengths, and plotting the graph of absorbance (y-axis) against wavelength (x-axis). In the application of Beer's Law, the best results will be obtained by using a wavelength corresponding to maximum absorbance of the sample. Note that wavelength is often denoted by the symbol lambda, λ, and the wavelength of maximum absorbance is then λmax. A spectrophotometer is an instrument that measures the absorbance (or % transmittance) of a solution at different wavelengths of incident light and will be used in this experiment. For instructions on the use of a spectrophotometer, see page 22 of this manual. Experimental Outline Aqueous iron Fe3+ solutions have a pale yellow colour with a relatively small molar absorbance coefficient (a). By contrast, when iron is reduced to the ferrous oxidation state (+2) and is complexed with an excess of 1,10-phenanthroline, a red coloured complex is formed whose molar absorbance coefficient is relatively large. The intensity of colour is stable, is independent of pH in the range of 2 to 9, and obeys Beer's Law. These criteria allow one to determine the concentration of iron in the concentration range of 0.02 to 4 mg/L directly; higher concentrations can be determined by dilution of the sample. The iron solution must be in the ferrous state. The reducing agent hydroxylamine hydrochloride is used to reduce any ferric iron (+3) to the ferrous (+2) state. . - - 2 Fe+3 + 2 NH2OH HCl + 4 OH → 2 Fe+2 + N2 + 2 Cl + 6 H2O Standard solutions of iron treated with phenanthroline are prepared and one of these is used to obtain a spectrum to determine the wavelength of maximum absorbance. At this wavelength the absorbance readings are most sensitive to changes in concentration of the iron complex. Using this wavelength, the absorbances of the standard solutions are determined and plotted as a function of concentration (Figure 1). The absorbance of an iron sample of unknown concentration is measured and its concentration is determined from the plot. A “blank” solution – one containing no analyte (species being measured, in this case Fe+2) but containing all the reagents used in the standards – is used to correct for the absorbance by the reagents. Zero the instrument with the blank solution. See page 22 for operation of the spectrometer. 48 Safety This experiment requires safety goggles during the entire lab period! You are working with strong acid, extra caution must be used. Do not boil the acid, just gently heat it. Do not spill the acid when handling it! Immediately notify your TA of any spills or exposure to acid. Procedure 1. Accurately weigh (on an analytical balance, see page 21) one iron supplement tablet (a green Ferrous Gluconate tablet) and place it in a 150 mL beaker with some boiling stones. Add 20 mL (graduated cylinder) of 1 M sulfuric acid. Heat gently (do not boil) on a hot plate, in the fume-hood, with occasional stirring to dissolve the tablet (note that some of the coating does not dissolve). Cool the solution to room temperature. Gravity filter the solution quantitatively, with two rinsings of distilled water, into a 500 mL volumetric flask. Make up to the mark with distilled water, cap and invert several times. 2. Dissolve about 0.05 g (weighed accurately on an analytical balance) of pure ferrous ammonium sulphate [FeSO4·(NH4)2SO4·6H2O] in about 100mL of water and transfer the solution to a one litre volumetric flask. Add 10 mL (graduated cylinder) of 4.5 M sulfuric acid and dilute the solution to the mark. This solution will be called the stock iron solution and may be shared by two or more groups of students. Solutions of sodium acetate (C2H3NaO2), 1,10-phenanthroline (C12H8N2) and hydroxylamine hydrochloride (NH2OH.HCl) have been prepared for you. 3. Into five 50 mL volumetric flasks, pipette 2.00, 5.00, 10.00, 15.00 and 20.00 mL portions of the stock iron solution. Put about 25 mL of distilled water in a sixth flask to serve as the blank. Into a seventh volumetric flask, pipette 1.00 mL of the prepared unknown iron solution. 4. To each of the seven flasks add: 0.5 mL (using the plastic transfer pipette) of the hydroxylamine solution, 5 mL (dispensette) of the l,l0-phenanthroline solution, and 4 mL (dispensette) of the sodium acetate solution. Dilute all the solutions to the 50 mL marks, cap and invert the flasks at least 10 times; then allow them to stand for ten minutes. 5. Using the blank as the reference and any one of the diluted standard iron solutions prepared above, measure the absorbance at different wavelengths in the interval 400 to 525 nm, remembering to re-zero the instrument for each wavelength. Take readings about 25 nm apart to find the region of maximum absorbance; then use intervals of 5 nm to find the maximum. Select the proper wavelength (λ of maximum absorbance) to use for the determination of iron with 1,10-phenanthroline and set the spectrometer to that wavelength. 6. Using the selected wavelength obtained in step 5, measure the absorbance of each of the standard solutions and the unknown. (The blank is used to adjust the meter to zero absorbance in step 4 of the operating instructions). Waste Unused stock iron solution may be poured down the drain. All other solutions must be disposed of in the provided waste beaker. 49 50 Laboratory E Spectrophotometric Determination of Iron PRELAB ASSIGNMENT page 1 of 1 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ 1. 2. If 0.1034g of ferrous ammonium sulphate hexahydrate is dissolved in water and made up to 1L of solution in a volumetric flask: a) What mass of ferrous ion is present in 1L of this solution _____________ g b) What is the iron concentration of this solution _____________ mg/L If 3 mL of the solution from #1 above is pipetted into a 100 mL volumetric flask and diluted with water to the mark, what is the iron concentration in the flask (in mg/L)? 51 52 Laboratory E Spectrophotometric Determination of Iron REPORT SHEET Page 1 of 3 Student Name: _____________________ Date: ____________ Course: _______ Section: ____ Data and calculations: 1. Unknown number or brand/type of tablet analysed ___________ 2. Mass of ferrous ammonium sulfate used in making up standard iron solution. ____________ 3. Concentration of the Fe+2 in your standard solution in mg/L Show your work. ____________ 4. Record the absorbances measured at different wavelengths for one of your standard solutions. Wavelength (nm) Absorbance Wavelength (nm) Absorbance Using excel or another graphing program, plot your data as a spectrum – that is, plot absorbance vs. wavelength. Follow the guidelines given in the introduction to the lab manual for preparing a graph. Hand in a print out of your graph with the report. Determine the wavelength of maximum absorbance (λmax) __________ nm 53 Laboratory E Spectrophotometric Determination of Iron REPORT SHEET Page 2 of 3 5. Calculate the concentration and record the corresponding absorbance for each diluted calibration standards (show a sample calculation): Sample Concentration (mg/L) Absorbance at λmax blank a b c d e f unknown Using excel, plot the absorbance at λmax as a function of the concentration. Follow the guidelines given in the introduction to the lab manual for preparing a graph. Hand in a print out of your graph with this report. 6. Record the concentration of the unknown Sample solution, as determined from the graph, in the space to the right. Show your work. 54 _____________________ mg/L Laboratory E Spectrophotometric Determination of Iron REPORT SHEET Page 3 of 3 Student Name: _____________________ Date: ____________ Course: _______ Section: ____ 7. Iron tablet: report the mass of iron in the tablet in mg of iron. Show your work. _____________________ mg of iron Question: 1. Explain the purpose of the blank in this experiment. In general, what should the blank contain? Student signature: 55 56 Lab F. STANDARDIZATION OF A SODIUM THIOSULPHATE SOLUTION Introduction In a volumetric analysis it is necessary to have a solution of known concentration, called a standard solution, to react with a sample of unknown concentration. A standard solution may be prepared by the simple procedure of taking a known amount of solid dissolved in to a known volume, in which case the term primary standard solution is applied. Alternatively, it is very often the case that a direct measurement of the amount of material cannot be made, or is very difficult to make accurately, for example if the sample is a gas, a volatile liquid or a deliquescent solid. In this case, a solution must be made up with approximately the required concentration and the actual concentration must be determined by titration with a primary standard solution. The term secondary standard solution is applied in this latter instance. Hence: 1. a primary standard solution is prepared by direct measurements of the mass of solute and the volume of solution. 2. a secondary standard solution is a solution whose concentration cannot be determined directly from weight of solute and volume of solution; the concentration must be determined by analysis (standardization) of the solution itself. Sodium thiosulphate crystals have the formula Na2S2O3•5H2O when freshly crystallized, but the salt has a tendency to effloresce or lose some of its water of crystallization (a property that is increased in very dry areas such as the Okanagan Valley). Since the true formula for the crystals changes and is unknown, sodium thiosulphate cannot be used as a primary standard. It is, fortunately, straightforward to prepare a solution of thiosulphate with approximately the desired concentration and to standardize it against a primary standard solution such as potassium dichromate or potassium iodate. Experimental Method In this experiment a solution of thiosulphate made up to approximately 0.01 M from sodium thiosulphate crystals is provided for you to standardize. A primary standard solution of potassium iodate, KIO3, is made up and used to standardize the thiosulphate solution. Two chemical reactions are involved in the process. First, the iodate is reacted with excess iodide (i.e., iodate is the limiting reagent) in acid solution to produce iodine; the equation is KIO3 + 5KI + 3H2SO4 → 3I2 + 3K2SO4 + 3H2O (I) Second, the iodine produced is titrated with the sodium thiosulphate according to the equation 2Na2S2O3 + I2 → 2NaI + Na2S4O6 (II) The end point of the titration is detected by using starch indicator. Starch forms an intense blue coloured complex with iodine and the end point is detected by the disappearance of the blue colouration as the last trace of iodine is reacted. Note that because iodine itself is coloured (yellow) it can act as its own indicator for most of the titration; however, the yellow iodine colour fades so gradually that it cannot be used to detect the end point. A combination of iodine and starch are used in the titration, the starch indicator not being added until the iodine colour has faded considerably. The titration is the whole process from the initial addition of thiosulphate from the burette to the end point detected by the starch indicator. 57 Safety This experiment requires safety glasses during the entire lab period! When adding 1M H2SO4, work in the fume-hood with the sash down at the required level. Procedure Notes: a. Take care not to confuse the chemicals potassium iodate (KIO3), potassium iodide (KI), and iodine (I2). b. A potassium iodate solution is made up and then diluted. The titration is carried out on the diluted solution until agreement between readings is reached. c. To reduce the weighing error for the iodate sample, a more concentrated solution than required is first made up. This is diluted and the diluted solution is used in the standardization. Part A. Preparation of Concentrated KIO 3 Primary Standard Solution 1. In a weighing container provided by your TA, accurately weigh out about 1.3 g of potassium iodate (see page 21) and quantitatively transfer it to a 150 mL beaker using about 75 mL of distilled water (rinse the weighing container with water, then add to the beaker). 2. When the iodate is fully dissolved, the solution is quantitatively transferred to a 250 mL volumetric flask. The beaker is rinsed with small quantities of water which in turn are transferred to the volumetric flask. Make up the solution to the 250 mL mark. Cap the flask and invert it with shaking at least 10 times to ensure complete mixing of the contents. N.B. This solution, the concentrated standard iodate solution, will be used both in this experiment and Lab G. Retain the concentrated solution of KIO3 in an appropriately labelled flask for Lab G. Part B. Preparation of Dilute KIO3 Primary Standard Solution 1. The concentrated standard solution of KIO3 is diluted ten-fold as follows: Pipette 25.00 mL of the concentrated standard KIO3 solution into a clean 250 mL volumetric flask. Dilute to the mark with distilled water, stopper the flask and shake. This is the dilute KIO3 solution. Part C. Titration 1. In a beaker, obtain about 100 mL of the sodium thiosulphate solution provided. Rinse and fill a burette with this solution. 2. Pipette 25.00 mL of the dilute standard iodate solution (from Part B.) into a 125 mL Erlenmeyer flask. Weigh out about 0.2 g (top-loading balance, since accuracy is not crucial in this case) of potassium iodide and transfer it into the flask. In the fumehood, add about 20 drops of 1 M H2SO4 (plastic transfer pipette). A brown solution of iodine appears; see equation (I). 3. The iodine liberated in step 2 is titrated with the sodium thiosulphate solution in the burette as follows: a. Record the initial burette reading to 2 decimal places. b. Add the thiosulphate slowly with constant swirling to the Erlenmeyer flask until there is only a faint colour of iodine left (straw-coloured solution). c. Add 1 mL of starch solution (plastic transfer pipette); the solution will become dark blue. 58 d. Carefully (dropwise with swirling) add thiosulphate from the burette until the blue colouration just disappears; this signifies the end point of the titration. e. Record the final burette reading to 2 decimal places. 4. The titration (steps 2 and 3) is repeated until two titrations agree within 0.1 mL. Waste Important!! Retain the concentrated KIO3 solution for Lab G! Dispose of any unused dilute KIO3 solution in the provided inorganic waste container. All other solutions may be poured down the drain. Calculations 1. From the weight of potassium iodate, determine the number of moles of potassium iodate that were dissolved to make the concentrated standard solution. Determine the molarity of this solution: 2. Determine the concentration of the dilute potassium iodate solution: In this case the initial volume is the pipette volume; the final volume is that of the volumetric flask. 3. From the concentration of the dilute potassium iodate solution determine the number of moles of potassium iodate that were pipetted into the Erlenmeyer flask. Determine the number of moles of I2 that were released in the flask; the KIO3 is the limiting reagent. 4. Determine the number of moles of thiosulphate (S2O32-) that have been added to the flask to completely react with the liberated I2. Using the volume of added thiosulphate solution and the average titration volume, determine the concentration of the thiosulphate solution. This sodium thiosulphate solution is now standardised and can be used in Exp. G. 5. From the errors in the weighings, volumes of pipettes, volumetric flasks, and burettes, determine the experimental uncertainty in the concentration of the thiosulphate solution. 59 60 Laboratory F Standardization of Sodium Thiosulphate PRELAB ASSIGNMENT page 1 of 1 Student Name: ________________________Date: __________Course: _____Section: ______ 1. Clearly explain the difference between a primary and a secondary standard solution (in your own words) 2. Why are standard solutions used? 3. 1.3208 grams of potassium iodate is dissolved and diluted to a volume of 250.0 mL. 25.00 mL of this stock solution is then pipetted into a 250.0 mL volumetric flask which is then filled to the mark. What is the concentration of the second standard solution (in mole/L)? SHOW YOUR WORK NEATLY. 61 62 Laboratory F Standardization of Sodium Thiosulphate REPORT SHEET page 1 of 3 Student Name: ________________________Date: __________Course: _____Section: ______ Data: 1. Mass of potassium iodate: Sample & Weighing Container ________________________ Weighing Container ________________________ Sample ________________________ (If you tare the balance with the empty container, omit the first 2 lines) 2. Burette Readings (insert proper units into the column headings) Trial # #1 #2 #3 #4 Error Final Burette Reading Initial Burette Reading Volume Used Circle the trials that you have averaged and record average:_____________________ 63 Laboratory F Standardization of Sodium Thiosulphate Calculations (including error calculations): REPORT SHEET page 2 of 3 (SHOW YOUR WORK) 1. Molar concentration of concentrated KIO3 solution: _____________________ 2. Molar concentration of dilute KIO3 solution: _____________________ 3. Number of moles of KIO3 pipetted into Erlenmeyer flask: _____________________ 64 Laboratory F Standardization of Sodium Thiosulphate REPORT SHEET page 3 of 3 Student Name: ________________________Date: __________Course: _____Section: ______ 4. Number of moles of I2 released by KIO3 in flask: _____________________ 5. Number of moles of Na2S2O3 reacted with I2: _____________________ 6. Molar concentration of Na2S2O3 solution: _____________________ Student signature: 65 66 Lab G. VOLUMETRIC ANALYSIS FOR VITAMIN C (C6H8O6) Introduction Canadian supermarkets are filled with the greatest abundance and variety of food ever available to man. However, the consumer's shopping list includes more and more foods that have been grown or processed in some way that has included contaminants or additives. Contaminants are compounds, organic or inorganic, that are retained accidentally in marketed foods, for example pesticide residues on plants and antibiotics or hormones in meat. Additives on the other hand are substances purposely added to foods during their preparation or processing to assure longer shelf life, greater attractiveness, consistency, flavour or ease of preparation. Additionally, many of us take pills containing various drugs and chemicals to cure or prevent anything from the common cold to pregnancy. Because of the many additives and contaminants there is a need to test quickly and accurately the amount of a given chemical in a particular product. Experimental Method This experiment will quantitatively analyze a sample for Vitamin C. Iodine reacts with Vitamin C by an addition process as indicated by the reaction equation below: Vitamin C (C6H8O6) One mole of iodine reacts with one mole of Vitamin C. This is a stoichiometric ratio of 1:1. In this experiment a solution of Vitamin C is added to a solution containing excess iodine. The amount of iodine in excess is then determined by titration with the standardized sodium thiosulphate. Safety This experiment requires safety glasses during the entire lab period! Do not crush the Vitamin C tablet with the glass rod, just gently stir. Procedure 1. In a clean 250 mL beaker, dissolve the tablet in 100 mL of distilled water with continuous stirring. 2. Stir thoroughly for several minutes to ensure that all the Vitamin C has dissolved (there will be an insoluble residue of 'filler'). Gravity filter into a 250 mL volumetric flask, washing the beaker and filter paper with small aliquots of distilled water, and make up to the mark with distilled water. Stopper and thoroughly mix the flask contents by inverting the flask at least 10 times. 3. Using the concentrated standard solution of KIO3 from Lab F, again make up a 250 mL of dilute standard solution of KIO3 following the instructions for Part B of Experiment F. 4. Rinse and fill a burette with the provided sodium thiosulphate solution. Note its concentration. 67 5. Pipette 25.00 mL of the dilute standard KIO3 solution into a 250 mL Erlenmeyer flask. Add 0.2 g KI (weigh on a top-loading balance). Add 1 mL of 1M sulfuric acid with a plastic transfer pipette (20 drops). Add 10.00 mL of Vitamin C solution with a pipette. 6. Record the initial burette reading. Titrate the contents of the Erlenmeyer flask, slowly, with stirring, until a pale yellow colour is obtained. Add 1 mL of starch solution and continue the titration until the blue colour just disappears. Record the final burette reading. 7. Repeat the titration at least twice more in order to obtain consistent results. Average the titration volumes. Data Record all burette readings. Also record the following with uncertainties: 1. 2. 3. 4. mass of KIO3 used to prepare the concentrated KIO3 primary standard solution Concentration of KIO3 in the concentrated standard solution Concentration of KIO3 in the dilute standard solution Concentration of Na2S2O3 calculated in Lab F Waste Dispose of any unused dilute KIO3 solution in the provided inorganic waste container. All other solutions may be poured down the drain. Calculations In this experiment, a known amount of iodine is produced in the Erlenmeyer flask. Vitamin C is added and reacts with some of the iodine. The remaining or excess iodine is then reacted with the sodium thiosulphate. 1. Determine the number of moles of I2 liberated by the KIO3 in the flask (from Lab F). 2. Determine the number of moles of sodium thiosulphate added to the flask, and from Equation (II), Lab F, determine the number of moles of I2 that reacted with the thiosulphate. 3. The difference in the amount of iodine liberated from calculation 1 and the amount reacted from calculation 2 is the amount of I2 that reacted with the Vitamin C. Since the reaction between Vitamin C and I2 is known (Equation (III)), then the number of moles of Vitamin C can be determined. 4. The number of moles of Vitamin C determined in calculation 3 is the Vitamin C that is present in 10.00 mL of solution. Determine the number of moles of Vitamin C present in the whole solution. This last quantity is the Vitamin C content (in moles) present in the 250 mg tablet. 5. Determine the weight in mg of Vitamin C present in your sample and compare your results with the manufacturer's claimed Vitamin C content. Because Vitamin C deteriorates with time, particularly in solutions, manufacturers usually add more than the amount guaranteed on the package in order to lengthen the shelf life of the product. 6. Determine the experimental uncertainty of your analysis. Note: Vitamin C is C6H8O6 and 1 mg = 1 x 10-3 g 68 Laboratory G Volumetric Analysis for Vitamin C PRELAB ASSIGNMENT page 1 of 1 Student Name: ________________________Date: __________Course: _____Section: ______ 1. Write the balanced equation for the generation of iodine in this experiment. 2. Write the balanced equation for the reaction of iodine and sodium thiosulphate. 3. In this method of analysis what two compounds react with the iodine produced from KIO3? 4. If you miss the endpoint by adding too much titrant, will the calculated moles of vitamin C be too high or too low? Explain your answer. 69 70 Laboratory G Volumetric Analysis for Vitamin C REPORT SHEET page 1 of 4 Student Name: ________________________Date: __________Course: _____Section: ______ Data: Vitamin C Brand/Type ___________________________ Listed Vitamin C mass per tablet __________________ 1. Molar concentration of stock KIO3 solution: _____________________________ 2. Molar concentration of dilute KIO3 solution: _____________________________ 3. Molar concentration of Na2S2O3 solution: _____________________________ 4. Burette Readings (make sure your table is complete – headings, units!) Trial # #1 #2 #3 #4 Final Burette Reading Initial Burette Reading Volume Used Circle the trials that you have averaged and record average:_____________________ 71 Laboratory G Volumetric Analysis for Vitamin C REPORT SHEET page 2 of 4 Calculations (including error calculations): (SHOW YOUR WORK) 1. Number of moles of I2 produced from 25 mL of the diluted KIO3 solution: ______________ 2. Number of moles of Na2S2O3 added in titration: ______________ 3. Number of moles of I2 used by the Na2S2O3 solution: ______________ 72 Laboratory G Volumetric Analysis for Vitamin C REPORT SHEET page 3 of 4 Student Name: ________________________Date: __________Course: _____Section: ______ 4. Number of moles of I2 used by the vitamin C solution: ______________ 5. Moles of vitamin C reacting with the I2: ______________ 6. Moles of vitamin C in the 10 mL sample: ______________ 73 Laboratory G Volumetric Analysis for Vitamin C REPORT SHEET page 4 of 4 7. Moles of vitamin C in the whole sample: ______________ 8. Mass (mg) of vitamin C in the whole sample: ______________ Student signature: 74 Lab K. DETERMINATION OF WATER HARDNESS Introduction As rainwater falls through the atmosphere, it becomes saturated with carbon dioxide and it becomes weakly acidic (pH = 5.6). When this acidic water percolates through the soil or runs over rock surfaces or through cracks in the rock, it dissolves limestone (CaCO3) and other substances. If the water accumulates an abundance of dissolved calcium, magnesium, or iron ions, it is called "hard" water. Ground water, taken from deep wells, usually has a greater load of dissolved ions than surface water from a stream or lake. When hard water is used for domestic and industrial purposes, the dissolved ions can cause problems. Calcium and magnesium ions react with soaps during laundering to form insoluble precipitates which stick to clothing. They also reduce the effectiveness of the soap. Iron stains often appear on white clothing. When hard water is heated in a kettle or boiler, deposits (scale) form which reduce the efficiency of heat transfer. The deposits can also significantly reduce the flow of water in hot water pipes and eventually cause them to crack. The most common way to "soften" water is through ion exchange. In this method, the hard water is forced through a bed of zeolite, a natural aluminosilicate material, or through a bed of synthetic polymer beads. The undesirable ions in the hard water are held by the ion exchange material and less objectional ions such as H+ or Na+ are released to take their place. The ion exchange material is regenerated periodically by exposure to concentrated NaCl or acid solutions. The following table shows the classification of water according to hardness: Hardness (ppm CaCO3) <15 ppm 15 ppm - 50 ppm 50 ppm - 100 ppm 100 ppm - 200 ppm >200 ppm Classification very soft water soft water medium hard water hard water very hard water Although hardness is the result of the presence of several different ions in water, the concentration of all hardness ions in a sample is usually reported as though the hardness were due exclusively to CaCO3. The units for hardness are mg CaCO3/L, which is equivalent to ppm CaCO3 (ppm = parts per million). 1mg/1Lwater= 1ppm (approximately) Experimental Method In this experiment, the hardness due to Ca2+ and Mg2+, of several different water samples is determined by titration. In a titration, a solution of one reactant of known concentration (the titrant), is carefully added from a burette to a solution of another reactant whose concentration is to be determined. The titration is complete when exactly enough of the titrant has been added to completely react with the other reactant. An indicator signals the endpoint of the titration usually through a colour change. The titrant in this experiment is the disodium salt of ethylenediaminetetraacetic acid (Na2C10H14N2O8). The anion of this salt, commonly referred to as "EDTA", reacts with both Ca2+ and Mg2+ ions, under basic conditions, as follows (M2+ = Ca2+ or Mg2+): 75 Na2EDTAaq -----> 2Na+aq + EDTA2M2+ + EDTA2- -----> MEDTA Since disodium EDTA is a stable compound with a composition that does not vary, it can be used as a primary standard in this titration. When it is carefully weighed and dissolved to make a known volume of solution, its concentration can be calculated accurately and it is thus called a primary standard solution. The indicator for this titration is a solution of calmagite. This compound is red in the presence of Ca2+ and Mg2+ ions. As EDTA is added, Ca2+ and Mg2+ ions bond to the EDTA and the colour of the solution becomes purple and eventually blue. The blue colour, without a hint of red, indicates the end of the titration. Safety This experiment requires safety glasses during the entire lab period! Calmagite is highly staining on skin and clothing. Procedure 1. Prepare a standard solution of EDTA as follows: (a) Accurately weigh about 1.0 g of disodium EDTA dihydrate (on an analytical balance). Quantitatively transfer this to a 250 volumetric flask, then add distilled water until the level is several centimetres below the neck. Swirl the flask frequently to dissolve the salt (note: this may take 10-15 minutes - go on with other parts of the procedure). (b) When the salt has completely dissolved, add distilled water to the flask until the bottom of the meniscus is at the mark on the neck of the flask. Cap the flask securely and invert it at least 10 times to insure complete mixing of the solution. 2. Obtain a burette and rinse and fill it with your standardized EDTA solution. Water from two different sources should be analyzed. These will be provided in the lab. 3. Obtain about 200 mL of water to be analyzed for hardness in a clean dry beaker. Pipette 50 mL of this water into a 250 mL Erlenmeyer flask. Using a graduated cylinder, add about 50 mL of distilled water to the flask. Then add 2 mL of calmagite indicator solution with a marked plastic pipette. 4. When you are ready to begin a titration, add 1.5 mL of the ammonium chloride/ammonia buffer solution to your sample. Mix this thoroughly and carry out the titration. For best results you must complete the titration within 5 minutes from the time the buffer is added. Repeat the titration for two additional samples. 5. Repeat steps 3 and 4 for a second water source. 76 Laboratory K Hardness of Water PRELAB ASSIGNMENT page 1 of 1 Student Name: _____________________ Date: ____________ Course: _______ Section: ____ 1. What is titrated in this experiment and what is the titrant? 2. What is the mole ratio between the hardness ions and the disodium salt of ethylenediaminetetraacetic acid? 3. Calculate the weight of calcium carbonate that contains 0.00021 moles of calcium. 77 78 Laboratory K Hardness of Water REPORT SHEET Page 1 of 2 Name _____________________ Course __________ Section _____ mass Date _________________ error Weighing Container EDTA+ Container EDTA Volume EDTA solution Molarity of EDTA (Show calculation of molarity including uncertainty) Sample A = _____________ Sample B =______________ Samples selected for analysis: Titration of Sample A: (circle all runs used) Final volume EDTA Initial volume EDTA Volume of titrant Run 1 Run 2 Run 3 Average Volume EDTA ______________ Titration of Sample B: (circle all runs used) Final volume EDTA Initial volume EDTA Volume of titrant Run 1 Run 2 Run 3 Average Volume EDTA ___________ 79 Laboratory K Hardness of Water Hardness: REPORT SHEET Page 2 of 2 Sample A = _______________ water Sample B = _______________ water (Show calculation for Sample A below) Sample A Sample B Volume of water pipetted Average volume of EDTA titrated Moles of EDTA titrated Moles of Caequiv. (Ca2+ + Mg2+) in water Moles of CaCO3 in water Mass of CaCO3 in water Hardness of water Note: neither the number of moles of Ca2+ nor the number of moles of Mg2+ is known, only their sum so we use Caequiv. to report the hardness as if it wre entirely CaCO3 Calculation for Sample A (Include error): Student signature: 80 Lab W. THE MOLAR VOLUME OF NITROGEN Introduction The volume of one mole of an ideal gas can be calculated for the atmospheric pressure and temperature conditions found in a laboratory. A real gas, however, does not follow ideal behaviour. Its molar volume must be determined experimentally. The difference between the ideal and experimental molar volume is called the deviation from ideal behaviour. In this experiment the volume of a known amount of nitrogen gas will be determined and compared to the calculated volume of the same amount of an ideal gas. The nitrogen gas will be generated from a known amount of sulfamic acid (HSO3NH2). NaNO2(aq) + HSO3NH2(aq) → NaHSO4(aq) + N2(g) + H2O Experimental The apparatus is assembled as shown in Figure 1. Safety This experiment requires safety glasses during the entire lab period! Use gloves when handling sodium nitrite and sulfamic acid. After each run, cap the suction flask with a rubber stopper and block the short side arm with a Pasteur pipette bulb, then take the flask to the fume-hood and empty the solutions into a waste beaker. 81 Procedure 1. Sulfamic Acid Solution (HSO3NH2) - In a small beaker (l00-l50mL), weigh out 1.35 to 1.50 g of sulfamic acid to an accuracy of ±0.0l g. (Which balance should you use?) Add about 50 mL of distilled water and stir to dissolve the solid. Quantitatively transfer the solution to a 100 mL volumetric flask and dilute with water to the line on the flask. Stopper and shake the flask. 2. Sodium Nitrite Solution (NaNO2) - In a small beaker (50-100 mL), weigh out 1.20 to 1.35 g of sodium nitrite to an accuracy of ±0.0l g. Add about 10 mL of distilled water and stir to dissolve the solid. Once the solid is completely dissolved, quantitatively transfer the solution to a 25 mL volumetric flask and dilute with water to the line on the flask. Stopper and shake the flask. 3. The reaction is run in the Gas Collecting Apparatus shown in Figure 1. A pipette is used to transfer 10.00 mL of the sulfamic acid solution to the suction flask. A pipette is used to transfer 3.00 mL of the sodium nitrite solution to a small vial. Using tweezers, the vial is carefully placed inside the suction flask along the side so that the vial won't tip over until the flask is shaken. The system is closed with a rubber stopper and the initial burette reading is taken. The flask is then shaken to start the reaction. After the gas evolution ceases, the final burette reading is taken. The temperature of the water and barometric pressure is also recorded. Calculations: 1) Moles of Sulfamic Acid in Reaction Flask a) From the mass of sulfamic acid, calculate the moles of sulfamic acid placed into the 100 mL volumetric flask. b) Calculate the moles of sulfamic acid in the 10.00 mL that was pipetted into the reaction flask. 2) Moles of Sodium Nitrite in Reaction Flask a) From the mass of sodium nitrite, calculate the moles of sodium nitrite placed into the 25 mL volumetric flask. b) Calculate the moles of sodium nitrite in the 3 mL that was pipetted into the reaction flask. 3) Limiting Reagent and Moles of Nitrogen a) Using the balanced equation and the results from 1 b) and 2 b), determine which reagent is the limiting reagent. b) Using the balanced equation, calculate the moles of nitrogen evolved from the limiting reagent. 4) Partial Pressure of Nitrogen a) The partial pressure of nitrogen equals the total barometric pressure minus the vapour pressure of water and minus the pressure of the water remaining in the burette. The vapour pressure of water can be found in Table 1 given the reaction temperature. (A negligible error of up to 0.4% is introduced into the pressure by not equalizing the water levels when reading the gas volumes.) PH2O = dH2O x g x hH2O, 82 where h is the height of the water remaining in the burette Table 1. Vapour Pressure of Water Temp. (°C) Pressure (Torr) Temp (°C) Pressure (Torr) 10 9.2 20 17.5 11 9.8 21 18.7 12 10.5 22 19.8 13 11.2 23 21.1 14 12.0 24 22.4 15 12.8 25 23.8 16 13.6 26 25.3 17 14.5 27 26.7 18 15.5 28 28.3 19 16.5 29 30.0 5) Experimental Volume a) From the water temperature (expressed in K), the partial pressure of nitrogen and the volume data, calculate what the experimental volume would be at STP using the ideal gas relationship P1V1 /T1 = P2V2 /T2. 6) Molar Volume of Nitrogen a) From the experimental volume of nitrogen at STP from 5 and the moles of nitrogen evolved from 3 b), calculate the experimental molar volume of nitrogen in litres/mole. 7) Calculate the molar volume of an ideal gas at STP in litres/mole. 8) Calculate the % deviation from the ideal behaviour : (experimental molar volume) - (ideal molar volume) x 100% ideal molar volume 83 84 Laboratory W The Molar Volume of Nitrogen PRELAB ASSIGNMENT page 1 of 1 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ 1. In this experiment, why do we have to include the vapour pressure of water in the calculation for the partial pressure of nitrogen? 2. If we were to react 0.146 g of sulfamic acid with 0.120 g of sodium nitrite, what volume (mL) of gas would we expect to be produced at standard temperature and pressure? 85 86 Laboratory W The Molar Volume of Nitrogen REPORT SHEET page 1 of 3 Name: ____________________________Course: ________ Section: ______ Date: ____________ DATA 1. Sulfamic Acid : 2. Sodium Nitrite: mass of beaker plus HSO3NH2 ______________g mass of beaker ______________g mass of HSO3NH2 ______________g mass of beaker plus NaNO2 ______________g mass of beaker ______________g mass of NaNO2 ______________g 3. Water temperature ______________oC 4. Barometric Pressure ______________torr 5. Volumes of Nitrogen Run 1 2 3 Final Volume __________ __________ __________ __________ mL Initial Volume __________ __________ __________ __________ mL Total Volume __________ __________ __________ __________ mL Average volume of nitrogen 6. Vapour Pressure of Water 4 _____________ mL _____________ torr 87 Laboratory W The Molar Volume of Nitrogen REPORT SHEET page 2 of 3 RESULTS Show your work for all calculations N.B. The numbering in this section corresponds to the numbering in the calculations section. 1. 2. 3. 88 a) Moles of HSO3NH2 in 100 ml volumetric flask. _____________moles b) Moles of HSO3NH2 in reaction flask. (10 ml) _____________moles a) Moles of NaNO2 in 25 ml volumetric flask. _____________moles b) Moles of NaNO2 in reaction flask. (3 ml) ____________moles a) Name the limiting reagent (write formula) _________________ b) Moles of N2 evolved. ____________ moles Laboratory W The Molar Volume of Nitrogen REPORT SHEET page 3 of 3 Name: ____________________________ Course: ________ Section: ______ Date: ____________ 4. Partial pressure of N2: ____________torr Temperature: Water Vapour Pressure: ____________°C __________K ____________torr Barometric Pressure: ____________torr Show your work: P N2 = 5. Experimental Volume at STP: ____________litres 6. Experimental molar Volume of N2 at STP: _____________ litres/mole 7. Percent Deviation from Ideal Behaviour _____________% Student signature: 89 90 Lab L. POLYMER CHEMISTRY Introduction A polymer (meaning "many membered") is a macromolecule made by chemically linking together many small molecules called monomers. The number of monomers that may be linked together can be small or as large as 10,000 units or larger. Polymers are classified in various ways: by their mode of synthesis, structure, properties, type of bonding that links the monomer units together, etc. The various classifications overlap each other such that a polymer can be better described using several classification systems. In this laboratory experiment you will study different polymer classification systems and you will prepare a selection of different types of polymers. Classification Systems 1. Mode of Synthesis Polymers are generally made by one of two synthetic modes: condensation polymerization or addition polymerization. a) Condensation Polymerization In this type of polymerization reaction, the polymer is made from the monomer with the elimination of a small molecule such as water, ammonia, hydrogen chloride, carbon dioxide, etc., as a side product. Examples of this type of polymerization would be the formation of Dacron (or Terylene) from terephthalic acid and ethylene glycol and the formation of nylon-6,5 from adipoyl chloride and cadaverine. 91 b) Addition Polymerization In this type of polymerization reaction the polymer is the only product formed. No side-products are formed. Examples of this type of polymerization reaction would be the formation of Nylon-6 from εcaprolactam and the formation of Orlon (Acrilon) from acrylonitrile. 2. Structure The structure of polymer molecules is classified as linear, planar or network (3-dimensional). The common industrial polymers are linear and network polymers. Planar polymers are mainly in the developmental stage at this time and are not common. 92 a) Linear Polymer A linear polymer is formed from monomers that are difunctional, that is, they have only two points of reactivity. The polymer that forms is usually quite flexible and can be softened upon heating. These polymers can be spun into fibers or molded into various shapes. If the shape is not suitable, the material can be resoftened and remolded, hence, the word 'plastic" is applied to these polymers. Examples of linear polymers would be Dacron, Nylon-6,5, Nylon-6, and Orlon as shown above. Other linear polymers have these trade names: Styrofoam, Lucite, Teflon, Polypropylene, Saran and Polyvinyl Chloride. b) Network Polymer A network polymer is formed from monomers that have more than two functional groups. Usually, they have three points of reactivity although four is not uncommon. The network polymer is a threedimensional array of bridges and connections forming a rigid structure that cannot be softened by heating or dissolved by solvents. Polymers of this type are also referred to as thermoset polymers. Note in the Bakelite example above, the phenolic group forms three links (tri-functional) while formaldehyde forms two (difunctional). Is this an addition or condensation reaction? The glycerol in the above example is trifunctional while the aromatic ring is difunctional. Would this reaction be an example of a condensation or an addition reaction? What is the criterion used to make this distinction? 3. Bonding Type Polymers are also classified by the type of bond that forms which connects the monomers. Examples would be Polyester, Polyamide and Polyolefin. a) An ester bond is formed when a carboxylic acid or its derivative combines with an alcohol to form an ester link. An example of the formation of an ester bond is found in part 1 a), the formation of Dacron. b) An amide bond is formed when a carboxylic acid or its derivative combines with an amine to form an amide link. 93 Examples of the formation of an amide bond is found in part 1 a), the formation of Nylon-6,5 and in part 1 b), the formation of Nylon-6. c) In the formation of a polyolefin, a double bond is broken from 1 monomer which can add to another monomer, etc. An example of a polyolefin bond is found in part 1 b) in the formation of Orlon. Safety This experiment requires safety goggles during the entire lab period! Procedure Station 1. Bakelite-type Polymer (Fume-hood) In a medium test tube, mix 1.5 mL of a saturated solution of aniline hydrochloride with 1.5 mL of formalin( 37% formaldehyde solution). (CAUTION: Do not get these chemicals on your skin; do not breathe their vapours.) Stir the solution well and record the change that occurs. Billiard balls and pot handles are made from Bakelite. Station 2. Alkyd Polymer – I (Benchtop) In a medium test tube, mix 2.0 g of phthalic anhydride, 1 mL of glycerol and 0.1 g of sodium acetate. Heat the mixture in a small Bunsen flame just to the boiling point until all the reactants are liquid, and then heat for 30 seconds more. (CAUTION: Heat sample very cautiously to prevent vigorous boiling. Do not point the test tube towards anyone when heating.) Set the liquid aside to cool slowly. Record the change that occurs. 94 Station 3. Alkyd Polymer – II (Benchtop) Use procedure for Alkyd Polymer - I, but substitute Ethylene Glycol for Glycerol. Station 4. Polymethylmethacrylate or better known as Plexiglas (Fume-hood) In a medium test tube, add 4 mL of methyl methacrylate and a pinch of benzoyl peroxide. Stir to dissolve the peroxide and warm the solution in a boiling water bath (100 mL beaker) for approximately ten minutes. Record the change that occurs. Station 5. Nylon-6,l0 (Polyhexamethylenediaminesebacoate) (Fume-hood) In a 100 mL beaker, add 5 mL of a 5% aqueous solution of hexamethylene-diamine (l,6diaminohexane) and 5 drops of 20% sodium hydroxide solution. Carefully add 5 mL of a 5% solution of sebacoyl chloride (decanedioyl chloride) in cyclohexane to the solution by pouring it down the wall of the slightly tilted beaker. Two layers will form, and there will be an immediate formation of polymer film at the liquid-liquid interface. With a copper wire hook, loosen the polymer from the sides of the beaker and slowly pull out the polymeric mass in a long rope. (Discard the spent solutions into the jars or Ziploc bags provided in the hood.) Do not handle the polymer with your hands. Nylon fibres are strong and they are used in clothing, ropes and parachutes. 95 6. The Fun Polymer: Slime™ aka Poly(vinyl alcohol) and Borax (Benchtop) An unusual example of cross linked polymer is that of poly(vinyl alcohol) (PVA) and Borax (sodium borate). Commercially, this is known as Slime™. As you’ll see (or already know) Slime has some very interesting properties. In essence, Slime is simply water that is held together by a cross-linked netting of borate and PVA. Much like a net holding oranges, the macro-physical properties are very different than its constituent parts. The polymer is actually 96% water and is only 4% polymerized material. The water is ‘held’ in the cross-linked netting by a combination of hydrogen bonding and the physical entrapment by the borate/PVA net. Add 10 mL of 4% PVA solution to a disposable medicine cup. Add a drop of food colouring (if provided) and mix in 2.5 mL of 4% Borate solution using a stir stick. Mix thoroughly. Once the ‘Slime’ begins to set it can be safely handled with bare hands. (Please dispose of it in the provided waste Ziploc bag when you are done. Rinsing it down the sink will plug the sinks.) Slime is used in special effects, for example in the film industry. Waste Test tubes should be rinsed with acetone into the organic waste container and discarded in the contaminated glass waste. If the polymer can’t be rinsed out, put the test tube in the provided Ziploc bag. Unused solutions should be disposed of in the provided beakers or the organic waste container. Polymer products go in the provided Ziploc waste bags. 96 Laboratory L Polymer Chemistry PRELAB ASSIGNMENT page 1 of 1 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ Questions: 1. Explain the difference between a condensation polymerization and an addition polymerization reaction. 2. The synthesis of Kevlar is illustrated below. Describe the following: • • • • • Mode of synthesis: ______________________________ Structure: _______________________________ Bonding type: _______________________________ H2N NH2 HN HO2C H N CO2H O O C C n + nH2O 97 98 Laboratory L Polymer Chemistry Student Name_________________ REPORT SHEET page 1 of 2 Course________Section_____Date_______________ A. Describe the changes observed during the polymerization reactions and the physical appearance of each polymer. 1. Bakelite-type Polymer: 2. Alkyd Polymer-I: 3. Alkyd Polymer-II: 4. Polymethylmethacrylate: 5. Nylon-6,10: B. How would you expect the physical properties to differ between Alkyd Polymer-I and Alkyd Polymer-II? Explain why these properties differ between the two polymers. C. Would it be correct to describe polymethylmethacrylate as a polyester? Explain your reasoning. 99 Laboratory L Polymer Chemistry REPORT SHEET page 2 of 2 D. Complete the table by placing a check in the box under the description which applies to the polymer. Polymer 1. Bakelite-type 2. Alkyd-I 3. Alkyd-II 4. Polymethylmethacrylate 5. Nylon-6,10 Condensation Addition Linear Network Polyester Polyamide Polyolefin E. In the space below draw the structure of the monomers required to make the given polymer by the mode of synthesis stated. 1. 2. Student signature: 100 Lab N. THERMOCHEMISTRY (CALORIMETRY) Introduction In general, when a chemical reaction occurs, heat is either evolved from the system into the surroundings or the opposite occurs. Those reactions in which heat is evolved are known as exothermic reactions, and, by convention, heat evolved from a system is given a negative sign. Those reactions in which heat is absorbed by the system are known as endothermic reactions and the heat absorbed is given a positive sign. Under conditions of constant pressure, the transfer of heat is equal to the change in enthalpy, ∆H. Enthalpy is a state function; that is, the change in enthalpy during a process is independent of the path taken to go from the initial to the final state. For the reactions NaOH (s) + HCl (aq) →NaCl (aq) + H2O NaOH (s) → NaOH (aq) NaOH (aq) + HCl (aq) → NaCl (aq) + H2O ∆H1 (1) ∆H2 (2) ∆H3 (3) the final state of the system at the end of processes (2) and (3) is the same as the state of the system at the end of process (1). Hence we must have ∆H1 = ∆H2 + ∆H3 (4) This result was first described by Hess and subsequently became known as Hess's law. Hess's law was first stated as follows: the heat of a reaction is the same whether it takes place in one or several steps. It is now known that this is true only if the heat flow occurs under conditions of constant pressure or constant volume. However, in cases where neither the pressure nor the volume is kept constant, the flow of heat is not equal to a state function, meaning that it is dependent on the path chosen and the additive rule (4) may not apply. In this experiment the enthalpy changes in reactions (1), (2) and (3) above are to be measured and the validity of Hess's law tested under conditions of constant pressure. To do this, a calorimeter will be used. A calorimeter is an instrument used to measure the heat absorbed or evolved in a reaction. The instruments range from the very simple inexpensive model to highly sophisticated and precise instruments valued at several thousand dollars. 101 Experimental Method In Part A of this experiment, the heat capacity of the calorimeter (Ccal) will be determined through calibration. The heat capacity is the quantity of heat in Joules required to raise the temperature of the calorimeter by l K (or 1 °C). The calorimeter in this experiment will consist of double Styrofoam cups with lid, a stirring wire, and a thermometer as shown above. The heat capacity is determined as follows: the calorimeter is filled with water of known volume and temperature. Some hot water of known volume and temperature is added. The final equilibrium temperature is measured. Since the process has been carried out at a constant pressure and heat is not exchanged with the surroundings, we can write: Heat change by hot water + Heat change by cold water + Heat change by calorimeter = 0 (5) Since the quantity of heat associated with a change in temperature, at constant pressure, is given by the relationship ∆H = n Cp∆T for a substance like water or ∆H = Ccal ∆T for a vessel like the calorimeter, equation (5) can be rewritten as: n1Cp (T3 – T1) + n2Cp (T3 – T2) + Ccal (T3 - Tl) = 0 (6) where n1 and n2 are the number of moles of cold and hot water respectively, Cp is the molar heat capacity of water with a value of 75.31 J/mol·K, T3 is the final temperature, and T1 and T2 are the initial temperatures. Ccal in units of J/K can be determined from this equation. (Note: The temperature units for heat capacity are J/K. However, since only changes in temperature are used and a change of 1 K equals a change of 1 °C, °C may be used for all T values.) In Part B of this experiment, the change in enthalpy of reaction (2) will be measured. The calorimeter is filled with water of known volume and temperature. A known weight of NaOH (s) is added. The equilibrium temperature of the resulting solution is measured. Again, because the process was carried out at constant pressure with no heat exchanged with surroundings, Heat loss by reaction (2) + Heat gain by solution + Heat gain by calorimeter = 0 (7) which becomes ∆H2 + n Cp(T5-T4) + Ccal (T5-T4) = 0 (8) where ∆H2 is the change in enthalpy of reaction (2), n is the moles of water in the calorimeter, Cp is the heat capacity of the solution which, because it is dilute, can be assumed to be the same as that for water, T5 is the final temperature, and T4 is the initial temperature. The solution obtained from Part B is saved for Part C. In Part C of this experiment, the change in enthalpy of reaction (3) will be measured. The calorimeter is filled with a sodium hydroxide solution of known volume, concentration and temperature (from Part B). A hydrochloric acid solution of known volume, concentration and temperature is added. The equilibrium temperature of the resulting solution is measured. At constant pressure, Heat loss by reaction (3) + Heat gain by the solution + Heat gain by calorimeter = 0 (9) the enthalpy change ,∆H3,for reaction (3) is determined from ∆H3 + n Cp (T7 - T6) + Ccal (T7 - T6) = 0 (10) where n is the total moles of water in the calorimeter. (Treat the hydrochloric acid solution as pure water) 102 In Part D of this experiment, the change in enthalpy of reaction (1) will be measured. The calorimeter is filled with a solution of hydrochloric acid of known volume, concentration and temperature. A known weight of sodium hydroxide is added and the equilibrium temperature of the resulting solution is measured. Again, at constant pressure, Heat loss by reaction (1) + Heat gain by the solution + Heat gain by calorimeter = 0 (11) the enthalpy change, ∆H1, for reaction (1) is ∆H1 + n Cp (T9 - T8) + Ccal (T9 - T8) = 0 (12) (where n is the moles of water in the calorimeter.) Safety This experiment requires safety glasses during the entire lab period! Procedure Assemble the calorimeter as shown in Figure 1. Part A. Calibration of the Calorimeter 1. Pipette 50.00 mL (V1) of distilled water into the calorimeter and measure and record its temperature to the nearest 0.1°C (T1). 2. Pipette 50.00 mL (V2) of distilled water into a l00mL beaker and heat the water to about 40°C as measured using a second thermometer. This temperature is also recorded (T2). 3. The hot water is quickly poured into the calorimeter and the final equilibrium temperature is measured and recorded (T3). 4. Repeat procedure (a) - (c) once more to obtain two values of Ccal. The average Ccal value should be used in further calculations. It should be noted that Ccal may have a positive or negative value due to the magnitude of the experimental error. Part B. Measurement of Temperatures to Determine Enthalpy Change, ∆H2 1. Pipette 50.00 mL of distilled water into the clean, dry calorimeter and record the water temperature (T4). 2. Accurately weigh about 1 g of sodium hydroxide pellets into the weighing container provided by your TA. (Note: Sodium hydroxide readily absorbs both water and carbon dioxide from the air and so should be kept in a closed container at all times to minimize such reactions.) 3. Add the sodium hydroxide to the water in the calorimeter, replace the lid and stir the solution with the stirring wire until the pellets just dissolve. The maximum temperature is recorded (T5). Do not discard the contents of the calorimeter. Part C. Measurement of Temperatures to Determine Enthalpy Change, ∆H3 1. Use the calorimeter and contents from Part B. Allow the contents to cool to approximately their original temperature (ca. T4). Record the actual temperature (T6). 2. Pipette 50.00 mL of 0.550 M hydrochloric acid into a clean, dry beaker and adjust the temperature so that it is close to that of the solution in the calorimeter (T6). 3. Quickly pour the acid into the calorimeter and then record the maximum temperature (T7). 4. Repeat procedure for Part B and Part C once more to obtain duplicate results. 103 Part D. Measurement of Temperatures to Determine Enthalpy Change, ∆H1 1. Pipette 50.00 mL of 0.550 M HCl into the clean, dry calorimeter and record the temperature (T8). 2. Accurately weigh about 1 g of sodium hydroxide pellets into a weighing container provided by your TA. 3. Add the sodium hydroxide to the calorimeter and stir to dissolve. Record the maximum temperature (T9). 4. Repeat procedure for Part D once more to obtain duplicate results. Calculations 1. Determine the duplicate values of Ccal and then take an average. Also calculate the error in this quantity. The average Ccal value should be used in subsequent calculations. 2. From the data of Part B determine: ∆H2 for both runs, the ∆H2 per mole NaOH for each run, the average ∆H2/mole NaOH and its uncertainty. Carry out similar calculations for ∆H3 and ∆H1. 3. Use the average ∆H1/mole, ∆H2/mole and ∆H3/mole values to assess the validity of Hess's Law. 4. Suggest reasons for using ±0.5 mL as the error in the 50 mL volumes rather than ±0.05 mL which is the uncertainty of a 50 mL pipette. Note: When estimating the experimental error the following errors should be used: Error in 50 mL volumes, ±0.5 mL Error in temperature readings, ±0.l°C Note: In determining the errors in this experiment it is extremely important to apply the following rules: 104 * To determine the error in two quantities that are multiplied or divided, the relative errors in the two quantities are added. * To determine the error in two quantities that are added or subtracted, the absolute errors in the two quantities are added. Laboratory N Thermochemistry (Calorimetry) PRELAB ASSIGNMENT page 1 of 1 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ 1. Briefly explain why the heat capacity of the Styrofoam cup calorimeter must be calculated in this experiment. 2. If the styrofoam cup calorimeter leaks energy to the surroundings, how will the measured value of Ccal be affected? A. Too large B. No effect C. Too small 105 106 THERMOCHEMISTRY DATA Student Name: ______________________ Date: ____________ Course: _______ Section: ____ Insert appropriate column headings T1 T2 T3 T4 T5 T6 T7 T8 T9 Part B NaOH weight Part D NaOH weight 107 Laboratory N Thermochemistry (Calorimetry) REPORT SHEET page 1 of 3 THERMOCHEMISTRY CALCULATIONS Error calculations are required for this lab!! Ccal: n1Cp (T3 - T1) + n2Cp (T3 - T2) + Ccal (T3 - Tl) = 0 (6) Calculations for Ccal, Run 1: Ccal, Run 1 = ____________________ Ccal, Run 2 = ____________________ Cavg. = ____________________ (Use this value in remaining calculations.) ∆H2: ∆H2 + nCp(T5-T4) + Ccal (T5-T4) = 0 Calculations for ∆H2, Run 1: ∆Η2, Run 1 = ___________________ 108 ∆Η2, Run 2 = ___________________ (8) Laboratory N Thermochemistry (Calorimetry) REPORT SHEET page 2 of 3 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ moles NaOH, Part B run 1 = ___________ ∆H2/mole1 = __________________ moles NaOH, Part B run 2 = _______________ ∆H2/mole2 = ___________________ ∆H2/moleaverage = ________________ ∆H3: ∆H3 + n Cp (T7 - T6) + Ccal (T7 - T6) = 0 Calculations for ∆H3, Run 1: (10) ∆H3, Run 1 = ___________________ ∆H3, Run 2 = ___________________ moles NaOH, Part B run1 = _______________ moles NaOH, Part B run2 = _______________ ∆H3/mole1 = __________________ ∆H3/mole2 = ___________________ ∆H3/moleaverage = ________________ ∆H1: ∆H1 + n Cp (T9 - T8) + Ccal (T9 - T8) = 0 (12) Calculations for ∆H1, Run 1: 109 Laboratory N Thermochemistry (Calorimetry) REPORT SHEET page 3 of 3 ∆H1, Run 1 = ___________________ moles NaOH, Part D run1 = _________________ ∆H1, Run 2 = ___________________ moles NaOH, Part D run2 = _________________ ∆H1/mole1 = ______________________ ∆H1/mole2 = ______________________ ∆H1/moleaverage = ___________________ Hess’s Law: ∆H1/moleavg = ______________________ ∆H2/moleavg + ∆H3/moleavg = ___________________ Was Hess’s Law obeyed (within experimental error)? Student Signature: 110 Yes No (Circle answer based on your observation) Lab P. THE DETERMINATION OF THE SOLUBILITY PRODUCT CONSTANT FOR CALCIUM IODATE Introduction Through common experience, we all have a working understanding of solubility. Take table salt (NaCl) for example. We can all appreciate that table salt dissolves in water. We may also realize that NaCl dissolves much better (more quickly and to a larger degree) in hot water versus ice cold water. The solubility of a chemical compound is defined as number of grams of solute in 1L of saturated solution, or number of moles of solute in 1L of saturated solution. Solubility varies with temperature. As chemists, it is important for us to be able to quantify a material’s solubility. By understanding and being able to calculate how well a material dissolves, we can more easily predict how such a solution will react with other chemicals. This experiment is designed to illustrate the concepts of solubility using a readily available salt, calcium iodate. Calcium iodate (Ca(IO3)2) is a moderately soluble salt. Qualitatively this means we can dissolve a small amount of material in a litre of water. Unlike sodium chloride, which is extremely soluble (357 g/L) or silver chloride, which is extremely insoluble (1.9x10-3 g/L), calcium iodate is described as sparingly soluble. By convention, we discuss solubility in terms of the solubility product constant, Ksp. In this experiment, we are going to determine calcium iodate’s solubility product constant. To determine the Ksp, we must make a saturated solution and determine (or infer) the concentration of each of the ions when the saturated solution is at room temperature. The solubility product constant is calculated as described below in equation #2. Ca IO Ca ! 2IO# (1) $% &Ca ' &IO# ' (2) In this experiment, Ksp will be calculated for four distinct solutions. In all cases, the solid precipitate (Ca(IO3)2) is filtered from the solution, and a known amount of filtrate is titrated with a standard solution of thiosulphate to determine the iodate concentration. Two chemical reactions are involved in the process. First, the iodate is reacted with excess iodide (i.e., iodate is the limiting reagent) in acid solution to produce iodine; the equation is KIO3 + 5KI + 3H2SO4 → 3I2 + 3K2SO4 + 3H2O (3) Second, the iodine produced is titrated with the sodium thiosulphate according to the equation 2Na2S2O3 + I2 → 2NaI + Na2S4O6 (4) The end point of the titration is detected by using starch indicator. Starch forms an intense blue coloured complex with iodine and the end point is detected by the disappearance of the blue colouration as the last trace of iodine is removed. Note that because iodine itself is coloured (yellow), it can act as its own indicator for most of the reaction; however, the yellow iodine colour fades so gradually that it cannot be used to detect the end point. A combination of iodine and starch are used in the titration, the starch indicator not being added until the iodine colour has faded. Finally the amount of iodate and calcium that was in the saturated solution is calculated. To calculate the iodate concentration, equations 3 and 4 must be added together in such a way as to give the same number of moles of I2 on both sides. This then gives the stoichiometric ratio of thiosulphate to iodate. 111 Safety: This experiment requires safety glasses during the entire lab period! Use gloves! Procedure: 1. If lab burettes are not set up, use clean, dry beakers to obtain aliquots of the stock solutions: about 65 mL of 0.075 M CaCl2 and about 110 mL of 0.075 M KIO3. 2. Using burettes make up the following four mixtures in 125 mL Erlenmeyer flasks. Solution A Solution B Solution C Solution D 0.075M CaCl2 20.00 mL 15.00 mL 12.50 mL 10.00 mL 0.075M KIO3 20.00 mL 25.00 mL 27.50 mL 30.00 mL 3. After each flask is completed, set it aside and scratch the inside of the glass wall with a glass stirring rod to initiate precipitation of the Ca(IO3)2. If this does not work, a seed crystal of Ca(IO3)2 may be placed into the Erlenmeyer. Once precipitation begins, set each flask aside for 10 minutes to ensure complete precipitation is achieved. Do not disturb the flask so the crystals can grow. 4. Using dry Buchner funnels and dry flasks, vacuum filter off the Ca(IO3)2 precipitates. Be very careful not to allow any solid Ca(IO3)2 to pass through the filter. You now have saturated solutions of aqueous calcium iodate. The iodate concentration in each solution will be determined by direct titration with standard thiosulphate solution. Obtain 150 mL of 0.1M sodium thiosulfate (Na2S2O3; make sure to note the exact concentration) in a clean beaker and rinse and fill a 50mL burette with some of this solution. 5. Pipette 10.00 mL of solution A into each of two flasks and add 1.0g potassium iodide (KI) to each. Once all the KI is dissolved and you are ready to titrate, add 1.0mL of 1.0M H2SO4 to the Erlenmeyer and begin titrating with thiosulfate until the solution takes on an orangereddish color. At this point the rate of thiosulfate addition should be reduced as the solution becomes more of a yellow color. Once the solution is a straw yellow color, add 2-3 drops of starch solution. This will change the solution to a dark purple/black color. Continue titrating very carefully until the purple color is only faintly visible, and continue dropwise until the purple disappears. 6. Repeat the titrations with duplicate 10.00 mL samples of solutions B, C and D adding 1.0 g of KI and 1.0mL 1.0M H2SO4 to each. 7. When washing glassware, ensure the glassware containing Ca(IO3)2 precipitate is thoroughly cleaned, as the solid tends to stick to the bottom of the glassware. Waste All solutions may be poured down the drain. 112 Laboratory P The Determination of Solubility Product Constant for Calcium Iodate PRELAB ASSIGNMENT page 1 of 1 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ 1. Define Ksp. Why is it useful? 2. Clearly explain the role of KI in the determination of the endpoint of the iodate ion titration. 3. What is the stoichiometric ratio of thiosulphate to iodate ion in the titration. 113 114 Laboratory P Solubility Product Constant For Calcium Iodate REPORT SHEET page 1 of 2 Student Name_____________________________Course________Section_____Date___________ DATA TABLE Volume of 0.075 M Ca(Cl)2 (mL) A B C D 20.00 15.00 12.50 10.00 B C D Volume of 0.075 M KIO3 (mL) Total volume of mixture (mL) Volume of mixture pipetted Volume of M Na2S2O3 titration 1 titration 2 AVERAGE: CALCULATIONS* A a) Total moles Ca2+ added in preparing each mixture b) Total moles IO3added in preparing each mixture c) Concentration of IO3- at equilibrium with Ca(IO3)2 (s) from titration data d) Total moles IO3(dissolved) in total volume of mixture 115 Laboratory P Solubility Product Constant For Calcium Iodate CALCULATIONS* REPORT SHEET page 2 of 2 A B e) Total moles of Ca(IO3)2 (s) precipitated out f) Total moles of Ca2+ (dissolved) in total volume of mixture g) Concentration of Ca2+ at equilibrium with Ca(IO3)2(s) h) The solubility product constant: Ksp (Ca(IO3)2) *Show details of calculation for mixture A only. Attach a separate sheet if necessary. Student Signature: 116 C D Lab Q. CHEMICAL EQUILIBRIUM: LE CHÂTÉLIER'S PRINCIPLE Introduction Chemical equilibrium is established when the rate of a chemical reaction occurring in one direction is equal to the rate of the same chemical reaction occurring in the reverse direction. This situation is called a dynamic equilibrium. Consider the general case where A, B, C, and D are chemical species and a, b, c, d are stoichiometric coefficients (numbers): aA + bB cC + dD (1) When equilibrium is established the forward and reverse rates are equal such that: c (2) d [C ] [ D ] a b [ A] [B ] = K c where Kc = equilibrium constant, [A] = concentration of species A in moles/L, and so forth. Kc has a constant value as long as the temperature remains constant. The subscript c means "concentration" indicating that the equilibrium constant in question is a ratio of concentrations as shown in (2). Le Châtélier’s Principle In 1884, Henri Le Châtélier pointed out that if a system at equilibrium is subjected to a stress that momentarily causes the system to be not at equilibrium, a spontaneous change will occur to bring the system back to equilibrium. This spontaneous change is always in the direction opposed to the original stress. This stress may be a change in temperature, concentration, or pressure in the case where gases are involved. The two stresses to be studied in this experiment will be changes in concentration and changes in temperature. Change in Concentration. Consider what would happen to equilibrium (1) if the concentration of A were suddenly increased. This would cause the quotient of concentrations as shown in equation (2) to be no longer equal to Kc. The equilibrium system will spontaneously remedy this situation because the increase in concentration of A would cause the forward rate of reaction to increase. This means that the rate of conversion of A and B to C and D as shown in equation (1) will be larger than the rate of conversion of C and D to A and B. The increase in concentration of A causes the equilibrium position to shift to the right to reduce the concentration of A as predicted by Le Châtélier. Equilibrium will be established when the quotient of concentrations shown in (2) are again equal to Kc. Change in Temperature. The effect of temperature upon Kc depends upon the sign of the enthalpy change, ∆H, of the reaction. The change in Kc as a function of T and ∆H is shown in Table 1. Table 1. The Change in Kc as a Function of T and the Sign of ∆H. Sign of ∆H T Kc + Increases Increases + Decreases Decreases - Increases Decreases - Decreases Increases 117 This table can be loosely understood by thinking of heat as a component of the reaction. An exothermic reaction would have heat as a product and conversely an endothermic reaction would have heat as a starting material. Suppose the forward reaction (1) were exothermic; that is to say the enthalpy (∆H) of the reaction aA + bB → cC + dD has a negative sign, or the reaction produces heat. Exothermic: A + B →C + D + heat The reverse reaction would have to be endothermic. If the system (1) was stressed by the application of heat, Le Châtélier’s Principle predicts that an endothermic shift would occur (to the reagent side). Conversely, if system (1) were cooled, the reaction would shift to the right to ‘resist’ the reduction in heat (an exothermic shift). Safety Multi-station labs usually require both safety goggles and glasses! Experiments done in the fumehood require safety goggles. For the experiments that can be done on the bench students may use safety glasses. The small picture beside some procedures will show whether goggles are needed. Procedures In this experiment we will examine a number of equilibrium systems. It is important to understand that the general principles discussed above apply to all of them. Part A-1. Equilibrium Shifts due to Changing Concentrations - Chromate/Dichromate Equilibrium (Fume-hood, safety goggles) In an acidic solution, the chromate ion (CrO42-) forms an equilibrium system with the dichromate ion (Cr2O72-): 2CrO42-(aq) + 2H+(aq) Cr2O72-(aq) + H2O The position of this equilibrium can be easily shifted by adjusting the acidity of the solution. Procedure 1. Place 2-3 mL of 1M K2CrO4 solution into each of two large test tubes, A and B. Note the colour of the CrO42- ion. 2. To test tube A: add 2 to 3 drops of 6M HNO3 and observe the colour change; then add 6M NaOH dropwise until a colour change is observed. Note the colour of the Cr2O72- ion and explain your observations. 3. To test tube B: add 2 drops of 1M KCl and note whether there is any change in colour. Explain your results. Part A-2. Equilibrium Shifts due to Changing Concentrations Ferric Thiocyanate Complex Ion Formation (Benchtop, safety glasses) Iron(III) chloride reacts with potassium thiocyanate to form a blood-red solution of the ferric thiocyanate complex ion: Fe3+ + SCN- 118 [Fe(SCN)]2+ - How would this equilibrium shift with the addition of the following compounds? (a) Fe(NO3)3 (b) KSCN (c) KNO3 Procedure 5. Add 5 drops of 0.1M Fe(NO3)3 (note colour) to 5 drops of 0.1M KSCN (note colour) in a 50 mL beaker and dilute this by adding 10 mL of water. Observe the colour of the mixture. Using a graduated plastic pipette, transfer 1 mL of the solution into each of five test tubes, A, B, C, D, and E. Use A as a colour reference. 6. To B, add 2 drops 0.1M Fe(NO3)3 and compare the colour with A. 7. To C, add 2 drops 0.1M KSCN and compare the colour with A. 8. To D, add 2 drops 0.1M KNO3 and compare the colour with A. 9. To E, add 1 mL of H2O and compare the colour with A. Part B. Sparingly Soluble Salts Sparingly soluble salts include the family of ionic substances that have a low solubility in water. In saturated solutions of these salts, an equilibrium between two phases occurs. This equilibrium between the insoluble solid and the soluble ions in solution is a heterogeneous equilibrium. Equation (3) shows this type of equilibrium for AgCl. Because the concentration of the solid phase remains constant, the equilibrium constant expression (4) takes the following form: AgCl (s) Ag+ (aq) + Cl- (aq) + - Ksp = [Ag ][Cl ] = 1.6 x 10 -10 (3) o at 25 C (4) The constant Ksp is called the solubility product constant. In order for an equilibrium to be present, the solution must be saturated and be in contact with some of the solid phase. If one prepares a saturated solution of AgCl at 25oC, the ion concentrations are [Ag+] = [Cl-] = 1.3 x 10-5 M What is important here is that equation (4) is obeyed. The fact that the ratio of [Ag+]:[Cl-] is 1:1 only reflects how the saturated solution was made. The ions were added to the water in a 1:1 ratio by dissolving some solid AgCl. When HCl is added to the above solution, the concentration of the Cl- ion will change. The ratio of [Ag+]:[Cl-] will no longer be 1:1 and the equilibrium in equation (3) will shift to the left to produce more AgCl (s). Regardless of the source of Cl-, equation (4) is obeyed. By adding HCl to the saturated solution of AgCl the [Cl-] increases while the [Ag+] decreases. The common-ion effect describes this shift in equilibrium due to the change in concentration of one of the components of the equilibrium. The equilibrium represented by equation (3) can also be shifted to the right to cause a precipitate of AgCl (s) to dissolve. This can be achieved by decreasing one of the ion concentrations by some chemical reaction. The usual way a precipitate of AgCl (s) is dissolved is by the addition of ammonia, NH3, which decreases the [Ag+] and causes equation (3) to shift to the right until no more precipitate remains. The specific way that ammonia decreases the [Ag+] is by forming a silver-ammonia complex ion shown in equation (5). Ag+ (aq) + 2NH3 (aq) Ag(NH3)2+ (aq) (5) 119 Procedure 1. In a medium test tube, combine 1.5 mL of 0.10 M AgNO3 solution and 1.5 mL of 0.10 M HCl. Heat in a boiling water bath for 5-10 minutes, then take out and allow to cool. Centrifuge the cooled test tube, then use a plastic transfer pipette to transfer 1 mL of the clear supernatant liquid to each of two test tubes, A and B. Note: Accurate solution preparation is very important. 2. From the known solubility of AgCl, 1.90 x 10-3 g/L, determine the Ag+ and Cl- concentrations in the saturated AgCl solution and hence the Ksp for AgCl. 3. To the clear supernatant liquid in test tube A add 1 mL of 0.10 M HCl. Record your observations and explain them in terms of a shift in equilibrium. Calculate the ion product [Ag+][Cl-] and compare it with the Ksp for AgCl. 4. To the solution in test tube B add 1 mL of 0.10 M HNO3. Record your observations. Calculate the ion product [Ag+][Cl-] and compare it with the Ksp for AgCl. 5. To the test tube containing the remaining liquid and solid AgCl add 6 M NH3 (aq) dropwise until a change is observed, mixing well after each addition. Note whether or not the AgCl precipitate dissolves and explain your observations in terms of the equilibrium theory. Part C. Complex Ion Equilibria and Temperature Effects Complex ions are polyatomic ions. A complex ion can gain or lose other ions or molecules, called ligands, to form uncomplexed or different complex ions. We have mentioned the silver-ammonia complex ion in part B. Another complex ion example is the equilibrium between the Co2+ ion and its ligands H2O and Cl- as shown in equation (6). It should be noted that Co2+ in water exists as the complex ion [Co(H2O)6]2+. [Co(H2O)6]2+ (aq) + 4Cl- (aq) Pink [CoCl4]2-(aq) + 6H2O (6) Blue The concentration of the ligands Cl- and H2O can be varied to shift the position of equilibrium and cause a change in colour. Moreover, the equilibrium constant for this reaction has a significant temperature dependence where a change in temperature can cause a change in colour. We will use this change in colour as a function of temperature to predict the sign of ∆H and determine whether reaction (6) is exothermic or endothermic using the principles summarized in Table 1. Procedure Use fume-hood! 1. To each of two medium test tubes, add 1.0 mL of 1.0 M CoCl2 (aq). One of the test tubes will serve as a colour reference. 2. To the other test tube add 1.0 mL of concentrated HCl. Record your observations and indicate and explain any equilibrium shift. 3. To the same test tube add 1.0 mL water. Record your observations and indicate and explain any equilibrium shift. 4. Place the same test tube into a beaker of boiling water for a few minutes, then place it into a beaker of ice water. Record your observations and any shift in equilibrium. Comment upon your observations in terms of temperature changes, equilibrium shift and changes (increase or decrease) in the equilibrium constant and determine whether reaction (6) is exothermic or endothermic as written. 120 Laboratory Q Chemical Equilibrium: Le Chatelier’s Principle PRELAB ASSIGNMENT page 1 of 1 Student Name: __________________ Date: _________ Course: _____ Section: _____ 1. Why isn’t the concentration of water included in Kc? (Answer must be BRIEF !) 2. Consider the equilibrium: Sb3+(aq) + Cl-(aq) + H2O SbOCl(s) + 2H+(aq) a.) Does the amount of precipitate increase, decrease, or stay the same when a small amount of sodium chloride solid is added to the equilibrium mixture (assume a negligible volume change on adding the sodium chloride)? b.) Does the amount of precipitate increase, decrease, or stay the same when the equilibrium mixture is diluted with water? Why? 121 122 Laboratory Q Chemical Equilibrium: Le Chatelier’s Principle Name:_____________________ REPORT SHEET page 1 of 4 Course:___________Section:___________Date:______ Part A: Equilibrium Shifts Due To Changing Concentrations 1) Chromate/Dichromate Equilibrium: Write the equilibrium reaction: _________________________________________________________________ Test Tube A: Colour of CrO42- ion: ______________________ Colour of Cr2O72- ion: ____________ Explain the effect of NaOH addition in terms of equilibrium shift: (Explain does not mean describe: answer why, not what!!) Test Tube B: Colour of test tube B after addition of KCl ____________________ Did the equilibrium shift when KCl was added to K2CrO4? Explain: 2) Ferric Thiocyanate Complex Ion Formation: Write the equilibrium reaction: ________________________________________________________________________ Colour of the Fe3+ ion: ________________ Colour of the SCN- ion: _______________ Colour of the Fe (SCN)2+ complex ion: _______________________ Explain any shift in equilibrium (as noted by a colour change) in test tubes B, C, and D: Tube B: No shift To Left To Right (circle your answer) Explain: ______________________________________________________________ ______________________________________________________________ 123 Laboratory Q Chemical Equilibrium: Le Chatelier’s Principle Tube C: No shift To Left To Right REPORT SHEET page 2 of 4 (circle your choice) Explain:_________________________________________________________ ______________________________________________________________________ Tube D: No shift To Left To Right (circle your choice) Explain:__________________________________________________________ _______________________________________________________________________ In test tube E the concentrations of the 2 reactants and the product have all been reduced to half the original concentrations on the addition of 1 mL of water. When the equilibrium is re-established the colour of the Fe(SCN)2+ ion is less intense than the expected half intensity. Explain in terms of the following equilibrium expression: &Fe SCN ' K &Fe ' &SCN # ' Part B. Sparingly Soluble Salts Write the equilibrium reaction: _______________________________________________________________________ 1. The solubility of Silver Chloride is 1.90 x 10-3 g/L Calculate the following: (MW of AgCl = 143.32g/mole) [Ag+] in the saturated solution = ____________________moles / L [Cl-] in the saturated solution = _____________________moles / L Ksp for AgCl = ___________________________________ 2. Test Tube A: Did a precipitate form on the addition of 1 mL of 0.10 M HCl? _____________ Explain in terms of equilibrium shift: 124 Laboratory Q Chemical Equilibrium: Le Chatelier’s Principle Name:_____________________ REPORT SHEET page 3 of 4 Course:___________Section:___________Date:______ Calculate the following ion concentrations immediately after addition of 1mL HCl: [Ag+] in 2 mL of solution in Test Tube A = ___________________moles / L [Cl-] in 2 mL of solution in Test Tube A =_____________________moles / L Note: the [Cl-] from the AgCl solution can be neglected. Calculate Q, the ion product [Ag+][Cl-] in the solution: __________________ Does this exceed the Ksp for AgCl? In the light of these calculations explain your results. 3. Test Tube B: Did a precipitate form on the addition of 1 mL of 0.10 M HNO3?____________ Calculate the following ion concentrations immediately after addition of 1 mL HNO3: [Ag+] in 2 mL of solution in Test Tube B = ____________________moles / L [Cl-] in 2 mL of solution in Test Tube B = _____________________moles / L Calculate Q, the ion product [Ag+][Cl-] in the solution:__________________ Does this exceed the Ksp for AgCl? In the light of these calculations explain your results 4. When 6M NH3 was added to the AgCl precipitate what change was observed? Explain in terms of the equilibrium theory. 125 Laboratory Q Chemical Equilibrium: Le Chatelier’s Principle REPORT SHEET page 4 of 4 PART C. Complex Ion Equilibria Write the equilibrium reaction: ____________________________________________________________________ Record your observations and explain any equilibrium shift for procedures 1, 2 and 3: 5. Addition of conc HCl: Colour of solution after HCl addition: _______________ Equilibrium shift: No shift To Left To Right Explain: (circle your answer) 6. Addition of H2O: Colour of solution after H2O addition: _________________ Equilibrium shift: No shift To Left To Right Explain: (circle your answer) 7. Temperature effect: Colour of hot solution: ________________________ Equilibrium shift: No shift To Left To Right Colour of cold solution: _______________________ Equilibrium shift: No shift To Left To Right Write the equilibrium constant expression for reaction (6): (circle your answer) (circle your answer) Is the reaction (6) exothermic or endothermic in the forward direction? Explain in terms of temperature and equilibrium constant changes. 8. If silver nitrate were added to a solution of CoCl42- what changes do you expect to observe? Student Signature: 126 Lab S. ACIDS AND BASES: TITRATIONS Introduction This experiment will 1) investigate the variation of pH during an acid-base titration 2) examine the use of indicators in an acid-base titration 3) use an acid-base titration to analyze a sample of acetic acid (a.k.a. vinegar). During the titration process, the addition of a base to a sample of an acid in aqueous solution leads to the reaction H3O+ + OH- → 2 H2O and to a net removal of H3O+ from the acid solution, causing an increase in pH. A plot of pH against mL of base added shows a rapid change in pH around the equivalence point. At the equivalence point, the pH of the solution is that of the salt in water. The solution may be acidic, basic or neutral depending on whether or not the salt ions hydrolyze. A monoprotic acid (e.g. HCl) will have just one equivalence point; a polyprotic acid (e.g. H3PO4) will have an equivalence point for the removal of each proton by the base. It is the rapid change in pH over the region of the equivalence point which makes the equivalence point readily observable. With a pH meter, the equivalence point is obtained from the plot of pH vs. mL of base added, and is the center point of the elongated s-curve. With visual indicators, it is the point at which the indicator changes colour, provided the correct indicator is used. Indicators are weak acids or bases which have the property that the acid and conjugate base are different colours. That is, when a base converts from the acid form (HIn) into the conjugate base form (In-)by removal of a proton, a colour change occurs: HIn + OH- → colour I H2O + Incolour II The colour change of the indicator will indicate the equivalence point of a titration, provided the indicator is weaker than the acid or base in the main reaction. Table 1 lists some indicators, their colours, and the pH ranges of their colour changes. 127 1. A strong acid-strong base titration may use almost any indicator. 2. A weak acid-strong base titration must use an indicator that is considerably weaker than the weak acid. 3. A strong acid-weak base titration must use an indicator which is a considerably weaker base than the weak base. 4. A weak acid-weak base titration is never carried out in practice due to the difficulty in detecting the end-point. 5. If the reaction involves more than one proton transfer, an indicator for each equivalence point must be selected. Table 1. Acid-Base Indicators Colour Indicator low pH high pH pH Range Bromophenol Blue yellow blue 3.0-4.6 Bromothymol Blue yellow blue 6.0-7.6 colourless pink 8.4-10.0 Phenolphthalein Safety: This experiment requires safety glasses during the entire lab period! Procedure Part A. Detection of Endpoint with a pH Meter 1. Rinse and fill a burette with standardized NaOH solution (note the molarity on the bottle). 2. Calibrate the pH meter following the instructions on page 21. Your TA will also demonstrate the correct use of the instrument. Once the pH meter is calibrated, do not turn it off or the calibration will be lost. Carry out the following titrations. 3. Pipette 10.00 mL of the hydrochloric acid solution into a 100 mL beaker. Add a magnetic stirring flea and place beaker on a magnetic stirrer. Immerse the electrode of the pH meter in the solution. Distilled water may be added to the beaker to ensure that the tip of the electrode is covered (have your lab instructor check). Determine the pH of the solution. 4. Add the sodium hydroxide solution from the burette to the stirred acid solution taking readings of the pH after the addition of each 1 mL of NaOH (Note: Additions do not need to be exactly 1.00 mL; instead, record the exact volume added and the matching pH reading.) When the pH is rapidly changing (i.e., near the equivalence point), take a reading of the pH after each 0.2 mL of base added. Take readings up to about pH = 12.0. 5. Repeat the above titration (steps 3 and 4), replacing the hydrochloric acid with 5.00 mL of acetic acid, CH3COOH, in approximately 20 mL of distilled water. NOTE that the amount of NaOH required may be quite different from that required for the HCl solution. Part B. Detection of Endpoint with indicators Using the supplied visual indicators, carry out the following titrations. 128 1. Pipette 10.00 mL of the hydrochloric acid provided into a clean 125 mL Erlenmeyer flask. Add 3 drops of bromophenol blue indicator. Titrate the hydrochloric acid solution to the indicated end point with the standard sodium hydroxide solution from the burette. Record the volume of sodium hydroxide solution added. 2. Repeat the titration using, in turn, bromothymol blue and phenolphthalein indicators. 3. Repeat the above three titrations (step 1 and 2) using 5.00 mL of acetic acid in approximately 20 mL of distilled water and each of the three indicators. Calculations 1. Use the data from Part A to plot a separate titration curve for each of the two acids by plotting the pH as a function of the volume of base added. ( see example in introduction section) 2. Use the data from the pH meter titration to analyze the acetic acid. Acetic acid (HA) is a weak monoprotic acid which reacts as follows: HA + NaOH → NaA + H2O Acetic acid (HA), CH3COOH, has a molecular weight of 60.1 g/mole. Determine the concentration of acid in mole/L, convert this to g/L and, using the approximation that 1.0 L of solution weighs 1.0 kg, determine the acetic acid composition in `percent by weight'. (Percent is a ratio rarely used in chemistry when discussing solutions. Percent can be percent by volume or percent by weight. This can lead to confusion. It is common in the food industry to describe both ratios as simply ‘percent’. Vinegar for example is measured in % by weight yet ethanol is measured in % by volume. Therefore, be careful in assuming the meaning of ‘percent.’) 3. Using both pH meter and visual indicator titration data, calculate the concentration (molarity) of both acids analyzed. 129 130 Laboratory S Acids and Bases: Titrations PRELAB ASSIGNMENT page 1 of 1 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ 1. Complete the following table (using the indicators phenolphthalein, bromothymol blue, or bromophenol blue): Titration of pH at equivalence point Best indicator(s) to use strong acid with strong base weak acid with strong base strong acid with weak base 2. Calculate the concentration of a benzoic acid solution (weak monoprotic acid) if the equivalence point of a titration on 10.00 ml of benzoic acid was reached after adding 22.61mL of 0.1062 M NaOH? 131 132 Laboratory S Acids and Bases: Titrations REPORT SHEET page 1 of 2 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ Molarity of NaOH: _______________________ Titration of HCl with NaOH using visual indicators Titration of HCl with NaOH using a pH meter Volume of NaOH used with: Plot a graph of pH vs. volume of NaOH added. Bromophenol Blue ____________________ Indicate on the graph the ranges of pH where Bromothymol Blue ____________________ each visual indicator changes colour. Phenolphthalein ____________________ Calculate the molarity of HCl using the Bromothymol Volume of NaOH used to reach the equivalence Blue endpoint volume: point _______________ pH at the equivalence point ___________ Calculate the molarity of HCl using the Equivalence point volume: Titration of CH3COOH with NaOH using visual Titration of CH3COOH with NaOH using a pH indicators meter Volume of NaOH used with: Plot a graph of pH vs. volume of NaOH added. Bromophenol Blue ____________________ Indicate on the graph the ranges of pH where Bromothymol Blue ____________________ each visual indicator changes colour. Phenolphthalein ____________________ Volume of NaOH used to reach the equivalence Calculate the molarity of CH3COOH using the point _______________ endpoint volume from one of the indicators (circle pH at the equivalence point ___________ your choice): Calculate the molarity of CH3COOH using the Equivalence point volume: 133 Laboratory S Acids and Bases: Titrations REPORT SHEET page 2 of 2 1. Analysis of acetic acid from pH meter data. a) Required volume of NaOH for neutralization b) Molarity of NaOH used c) _______________________ _______________________ Calculate the % by weight of acetic acid. Show your work below. Neatly! 2. What visual indicator would be appropriate for each of the following titrations? a) HCl + NaOH ⇋ NaCl + H2O b) CH3COOH + NaOH ⇋ NaCOOCH3 + H2O ________________________ ________________________ 3. Explain your answer for 2(b). 4. Since indicators change colour over a 100-fold change in acidity (pH range of 2) how is it that indicators are at all accurate? Comment on this. Student Signature: 134 _ Lab U. KINETICS: THE INVESTIGATION OF THE RATE OF CHEMICAL REACTION This experiment which studies the rate of a chemical reaction will be carried out over two weeks. In the first week the effects of concentration and catalysis will be investigated. In the second week, we will observe the effect of temperature. Introduction In this experiment, the effect of concentration of reactants, temperature and a catalyst on the rate of the reaction between persulphate and iodide is to be investigated. The reaction is (NH4)2S2O8 → 2NH4+ + S2O82KI → K+ + IS2O82- + persulphate 2I- → iodide 2SO42- + sulphate I2 (I) slow reaction iodine The rate of the process is determined by measuring the time required to produce a fixed amount of iodine. This is done by adding to various mixtures of the reactants a fixed quantity of thiosulphate ion and a few drops of starch solution. The iodine formed by the above reaction immediately reacts with the thiosulphate according to the equation 2S2O32- + I2 → S4O62- + 2I- (II) fast reaction (S2O32-) is consumed. At this point, the further production of iodine leads to until all the thiosulphate the rapid formation of blue-coloured starch-iodine complex which thus serves as the indicator of the extent of the first reaction. Since the same amount of thiosulphate is added in each case, the time taken for the solution to turn blue after the mixing of the reactants in a series of trials is a measure of the rate of the reaction. When investigating the concentration effects, dilutions are carried out with salt solutions. This is to keep the ionic strengths constant, since the rate of the reaction can also depend on the ionic strength of the solution. The temperature effect on the reaction rate is studied by measuring the time taken for the blue colouration to appear in the reaction mixture after the temperature of the reactants has been raised by about l0°C. From this result an estimation of the activation energy for the process can be made. Safety This experiment requires safety glasses during the entire lab period! Week 1. Effect of Concentration and Catalyst Procedure Part A. Effect of Concentration 1. If lab burettes are not set up, use clean, dry beakers to obtain aliquots of the 0.20M potassium iodide (KI) and 0.10M ammonium persulphate ((NH4)2S2O8) solutions and set up your own burettes. 2. Three separate systems of different concentrations are to be investigated. Dispense the required amounts of solutions from burettes into the appropriate beaker according to the system being investigated, as shown in Table 1 below. Use a 10.00 mL pipette to add the 135 0.20M potassium chloride (KCl) and the 0.10M ammonium sulphate ((NH4)2SO4) into the appropriate beakers. Table 1. Experimental Volumes System 1 2 3 Beaker A (a 250 mL beaker) 20 mL I10 mL I- + 10 mL KCl 20 mL I- Beaker B (a 100 mL beaker) 20 mL S2O8220 mL S2O8210 mL S2O82- + 10 mL (NH4)2SO4 3. Pipette 25.00 mL of 0.0050 M sodium thiosulphate solution (Na2S2O3) into beaker A, followed by 3.00 mL of starch solution. Quickly add solution B to beaker A while stirring. Measure the time from this instant until the solution turns blue. 4. Repeat step 3 to give two results for each of the three systems. In each case, record the temperature of the first run for future use (take this measurement after mixing beakers A and B and stirring for several seconds). Part B. Effect of a catalyst 1. Follow the procedure for System 1 in Part A above. Add 3 drops of 0.02 M copper sulphate solution to beaker A prior to the addition of solution B. The temperature should be approximately the same as used in Part A and the time for the reaction is measured as before. Do this twice. 2. Repeat step 1, but this time, use 6 drops of the copper sulphate solution. Calculations 1) On the report sheet, transfer from your lab notebook the concentrations of the persulphate, iodide and thiosulphate solutions together with the times taken for each of the runs and the temperatures determined for the first run. 2) Calculate of the order of the reaction. The rate equation is: rate = kr [S2O82-]m[I-]n (1) where kr is the specific rate constant and m and n are the orders of the reaction with respect to persulphate, S2O82-, and iodide, I-. Rate is a measurement of quantity change with time and in this experiment rate = d[ I 2 ] d[ I 2 ] , where is the rate of change of [I2 ] with time. dt dt Therefore, rate = d[ I 2 ] = kr[S2O82-]m[I-]n dt The ratio of rates of systems 1 and 2 can be obtained. 136 (2) d[I 2 ] rate1 dt 1 = rate 2 d [ I 2 ] dt 2 (3) Because reaction (I) has not proceeded very far by the time the blue coloration appears, the initial rate of the reaction is constant, i.e. from (3) ∆[ I 2 ]1 d[I 2 ] rate1 dt 1 ∆t1 = = ∆[ I 2 ] 2 rate 2 d [ I 2 ] ∆t 2 dt 2 Since the initial [S2O32- ] is the same for each system, each system will have produced the same quantity of [I2] when the blue coloration appears. This means ∆[I2 ] is the same for each system. Therefore, from (4) ∆[ I 2 ]1 rate1 ∆t1 ∆t = 2 = rate2 ∆[ I 2 ] 2 ∆t1 ∆t 2 (4) (5) Since tinitial = 0 in each system: and so ∆t 2 t 2 = ∆t1 t1 (6) rate1 t 2 = rate 2 t1 (7) The value of n, the order of the reaction with respect to iodide, can be found by combining equations (2) and (7) for systems 1 and 2: 2− k r S 2 O8 1m I − 1n rate 1 t = = 2 (8) 2− m − n rate 2 t1 k r S 2 O8 2 I 2 [ [ ][ ] ][ ] Hence, [ ] [ ] t2 I− = − t1 I n 1 2 n [ ] [ ] I− = − I n 1 (9) 2 The concentrations [I-]l and [I-]2 have been selected to have a simple relationship and n may be determined by inspection; n should have an integer value. A similar approach using systems 1 and 3 will give a value for m. From your data determine the orders of this reaction. It should be noted that the concentrations of S2O82- and I- used in this calculation should take into account the dilution that occurs on mixing the various volumes of reactants together. 3) Evaluate of the rate constant. From (2) and (4) rate = ∆[ I 2 ] d[ I 2 ] = kr[S2O82-]m[I-]n = ∆t dt (10) 137 Since [I2]initial = t initial = 0, ∆[ I 2 ] [ I 2 ] final − [ I 2 ]initial [ I 2 ] final [ I 2 ] = = = t final − t initial t final ∆t t (where the subscript, final, has been dropped) Combining equations (10) and (11), [I ] rate = 2 = kr [S2O82-]m [I-]n t (11) (12) In applying equation (12), m and n are known; the concentrations of S2O82- and I- can be calculated (again allowing for dilution); the rate can be determined from [I2]/t, and so kr may be calculated. The nI value for [I2] is obtained from [I2] = 2 where nI2 is the number of moles of I2 that has been V produced by the time the solution turns blue and V is the volume (in litres) of the reaction mixture. The number of moles of I2 in turn can be determined from the number of moles of thiosulphate that have reacted; this is the same for each system. Determine kr for systems 1, 2 and 3 and quote the appropriate units. 4) For Part B, record your data on the report sheet. Week 2. Effect of temperature Procedure 1. The experimental procedure is identical to that followed in week 1. Only system 1 will be studied. The temperature is measured after mixing the reactants and stirring for several seconds. 2. Temperatures to be used are as follows. a. Use system 1 measurements for last week at temperature T. (Do not repeat at this temperature.) b. System 1 at T + 5°C (i.e. 27°C if last week was 22°C) c. System 1 at T + l0°C d. System 1 at T + l5°C e. System 1 at T + 20°C f. System 1 at T - 5°C g. System 1 at T - l0°C Note that the temperatures need not be exactly those listed, but must be measured and recorded. 3. Enter the results onto the table on the report sheet. Calculations The system studied is identical to system 1 in Part A apart from the temperature at which the reaction takes place. A comparison can be made between these systems. 138 Determine the value of kr, the rate constant, at the 7 temperatures. Comment on the generalization, "the rate of a chemical reaction is approximately doubled for each l0°C rise in temperature". The Arrhenius equation accounts for the temperature dependence of the rate constant, ln(kr) = ln A - Ea RT where: Ea = activation energy T = absolute temperature (K) R = the gas constant, 8.314 J•mol-1K-1 A = Arrhenius constant By plotting ln(kr) (y-axis) against 1/T (x-axis) a linear graph should be obtained with slope = -(Ea/R). Determine Ea for the reaction under study. 139 140 Laboratory U Kinetics: The Investigation of the Rate of Chemical Reaction Student Name: ___________________ 1. PRELAB ASSIGNMENT page 1 of 1 Date: _________ Course: ____ Section: ____ Why does the [I-] not change until after the reaction mixture turns blue? Circle the most complete answer. A. The I- is converted to I2 by the ammonium persulphate, so the [I-] decreases, until the reaction turns blue. B. The rate at which the I- is converted to I2 by the ammonium persulphate is much slower than the rate at which the Na2S2O3 reacts with the I2 . C. Any I- consumed by Reaction I is replaced immediately by Reaction II until all the Na2S2O3 has been consumed. Then the I2 produced reacts with starch to form the blue colour. D. The I- reacts with the starch to produce a blue colour, so the [I-] decreases after the blue appears. 2. How far has reaction (I) progressed when the solution turns blue? a) near the end b) near the beginning c) finished d) half way Circle the correct answer. 141 142 Laboratory U Kinetics: The Investigation of the Rate of Chemical Reaction Name: ______________________ REPORT SHEET Page 1 of 4 Course: ______ Section:_______Date___________ Data: Concentration of Stock Solutions [I-] = ________________ [S2O82-] = _______________ [S2O32-] = ______________ In the following table, calculate the concentrations of the ions at the beginning of the reaction, allowing for the dilution that occurs on mixing the solutions together. System - [I ] [S2O82-] [S2O32-] Time (Seconds) Run 1 Run 2 Average Temp. C o 1. Calculate the value of n in the space below and then round the answer to an integer. 2. Calculate the value of m in the space below and then round the answer to an integer. 143 Laboratory U Kinetics: The Investigation of the Rate of Chemical Reaction REPORT SHEET Page 2 of 4 3. Determine [I2] produced by reaction I (as measured by the Na2S2O3) when the colour change is observed. This value is the same for each system. 4. Determine the rate of reaction for each system, [I2]/t in units of Ms-1. Also determine the value for kr. System 1 2 3 Rate kr kr(average) = 5. What are the units for kr? Catalytic Data System 1 No catalyst added 3 drops Cu2+added 6 drops Cu2+added Time (Seconds) Run 1 Run 2 Average 6. Is the effect of this catalyst dependent on its concentration? Explain. 7. In what way does a catalyst influence the rate of a reaction? 144 Laboratory U Kinetics: The Investigation of the Rate of Chemical Reaction Name: _______________________ REPORT SHEET Page 3 of 4 Course: ______ Section: ________ Date: ______ Temperature Effects Data Table Run *I II III IV V VI VII Time (s) Temp. (oC) Temp. (K) * Run I is from last week’s data. Calculations: include units Run *I II III IV V VI VII Rate kr ln kr Temp-1 (K-1) 145 Laboratory U Kinetics: The Investigation of the Rate of Chemical Reaction REPORT SHEET Page 4 of 4 1 1. Draw a suitably labelled graph of your data. Calculate the slope ln(k r ) / and deduce Ea, T the activation energy. Show calculations below and attach your graph. Ea = ___________________ 2. Once the order of the reactants, the rate constant and the activation energy are known, the kinetics of the reaction have been characterized. From your data determine the following: a) The rate of the reaction at 25oC when [I-] = [S2O82-] = 0.50 M ___________ b) The rate of the reaction at 50oC when [I-] = [S2O82-] = 0.50 M Show calculation for (b) ___________ Student Signature: 146 Lab Z. THE PREPARATION OF ACETANILIDE Introduction Acetanilide is used industrially in the manufacture of medicinals and of dyes and also as a stabilizer for H2O2 solutions. In this organic synthesis, several important and common techniques will be used. The acetylation is carried out to convert the amine, aniline, to the solid product, acetanilide, which is isolated, purified and quantified. Purification is accomplished by using charcoal to remove coloured impurities and the important technique of recrystallization for more chemical purity. Due to the relatively low solubility of acetanilide in cold water (1 gm/185 mL) and high solubility in boiling water (1 gm/20 mL) water may be used as the solvent. Pure product is obtained on cooling while impurities remain in the solvent. Vacuum filtration is used to collect the product which is then dried and its mass determined. The correct identity of the product is confirmed by measuring one of its physical properties. For solids this is usually the melting point. An additional benefit of using the melting point (range) is that the observed melting range (that range of temperature over which both solid and liquid phases are present) is a sensitive and reliable indicator of product purity. Safety: Aniline is a very toxic compound; take care to avoid contact with skin. Acetic anhydride is a harsh, very irritating liquid to eyes and nasal membranes. It may also cause severe burns. If either compound is spilled on the skin wash thoroughly with soap and water. Consult M.S.D.S. for more information. Procedure 1. Pre-weigh a stoppered 250 mL Erlenmeyer flask on a top-loading balance. 2. In the fume-hood, obtain about 4 mL of aniline. Re-weigh the stoppered flask with aniline. 3. Return to the fume-hood. Add 30 mL of water to the flask, followed by 5 mL (a slight excess) of acetic anhydride in several small portions with swirling. Continue swirling for about 5 minutes, after which the stopper may be removed. The rest of the procedure may be carried out on the bench. 147 4. The crude acetanilide is now recrystallized from water. Add 100 mL of water and a boiling stone to the Erlenmeyer flask. Heat and swirl gently until all of the solid and oil have dissolved. 5. Allow the flask to cool slightly while you set up for gravity filtration. After cooling 5-10 minutes, add approximately 0.5 to 0.7g of charcoal. Do not add the charcoal to a very hot solution as frothing will occur (causing loss of product and a mess to clean up). Swirl the mixture and resume a gentle boil for a few minutes after charcoal addition. Caution: do not overheat or frothing will occur. 6. Gravity filter the hot solution into a clean 250 beaker using a powder funnel and a folded filter paper (see page 19). Keep the solution hot until all is poured into the funnel and have 40 50 mL of boiling distilled water on hand for washing if needed. Do not worry about charcoal remaining in the flask. 7. Allow the solution to cool slowly to room temperature, do not use an ice bath. This will take 30 to 45 min. Use this time to set up the vacuum filtration and clean up from the first part. 8. Finish off the cooling in an ice bath (5 to 10 min.). Make sure your filter flask is clamped to a retort stand, then set up a Buchner funnel and vacuum filter the purified acetanilide, washing the crystals with a small quantity of ice-cold water. Dry the product as much as possible by sucking air through the crystals. 9. Using a flat spatula, remove the filter paper with product and place it in a 250 mL beaker. Allow the product to air dry for at least 48 hours. When you return, scrape product off the filter paper, determine its mass and melting range. 10. Clearly label a beaker or test tube containing your product with your name, section number, product name, melting range, mass and % yield. Leave this in your section’s locker for your instructor to mark. 148 Laboratory Z Preparation of Acetanilide PRELAB ASSIGNMENT page 1 of 1 Student Name: ______________________ Date: ____________ Course: _______ Section: ____ 1. a). What is the theoretical yield of the product C if 0.050 moles of A and 0.20 moles of B are used in the following reaction? (MW of product C is 110 g/mole) A + B → C b). If you produce 4.6 grams of product C what is your percent yield? 2. List, in the correct order, the steps of a recrystallization procedure. a) b) c) d) vacuum filter to isolate product dissolve in minimal amount of hot solvent cool slowly to allow crystals to form gravity filter hot solution The correct order is: _____________________________________ 149 150 Laboratory Z Preparation of Acetanilide Student Name: _____________________ REPORT SHEET page 1 of 2 Section _______ Course_______Date: ________ The mark for this lab will include point for both the quantity and quality of product turned in and the following questions. 1. Fill in the following table using your values: Mass Aniline Acetic anhydride (Show your work) Number of moles 2. What is the limiting reagent? ______________________________________ 3. Calculate the theoretical yield of acetanilide using your data (show your work). 4. Consult a reference book and find the melting point of pure acetanilide (melting range). m. p. __________________________ 5. a) Record your yield (mass) of product. _______________________________ b) Calculate the % yield of your product ______________________________ c) Record the melting range of your product. __________________________ 151 Laboratory Z Preparation of Acetanilide REPORT SHEET page 2 of 2 6. Where do you think losses in yield have occurred? 7. What techniques (3) were used in the preparation to improve the purity of the Acetanilide? Student Signature: 152 Appendix A Guide to Scientific Citation and Referencing in Chemistry. (Adapted from The ACS Style Guide 2006)1* *This is a real reference! All sources used to write a laboratory report or to gather background information should be cited and listed in the reference section. Copying without citation it is considered plagiarism and may result in a failing grade or other penalties up to expulsion from university. Every case will be reported to the lab manager and the Dean’s office. If you are uncertain about what constitutes plagiarism or the consequences of such an offence, please see the university guidelines and regulations on this matter. Within chemistry there are many different styles for references; one of the most common and the one we would like you to use is the Journal of the American Chemical Society (JACS) style. In text citations Direct quotations are rarely used in chemistry papers. Therefore, it is important that all cited information is paraphrased (put into your own words). Numerical reference citations are placed after the period at the end of the sentence as superscript numbers. eg. Peter Pauson and Tom Kealy were the first chemists to publish the synthesis of ferrocene (bis(η5-cyclopentadienyl)iron(II)) in 1951.1 Reference citations should be consecutively numbered as they appear in the text (starting with 1). However, if you cite the same source more than once, do not give it a new number; use the original reference number. These numbers must match up to the reference number in the reference section. To cite more than one reference, list the numbers in ascending order separated with commas without spaces; if there is a consecutive series, use a dash. eg. The development of ferrocene derivatives with functional groups has led to the incorporation of these complexes into larger molecules including polymers.2,3,5-10 References The reference section should be a list of all books, journal articles and other material cited in the introduction, methods and discussion sections of your lab report. References appear in sequential order based on the order they appeared in the text. Different sources of information (journal articles, book, web pages etc.) are referenced in slightly different ways. If your source does not fit into the following categories, you may refer to “The ACS Style Guide” which is available in the library. Be careful to mimic italics, boldface, spaces, semicolons, commas and periods used for the references. Also, if a piece of information such as the volume number or edition number is not available, you do not include it. 153 Journal articles / Review articles Journal articles are referenced by listing the authors (the last name, followed by initials), the title of the journal (usually abbreviated, in italics), the year of publication (boldface), the volume number (italics), and the first page number. Multiple authors are separated by a semicolon. JACS style does not include the titles of the articles. Journal names can be abbreviated according to http://www.library.ubc.ca/scieng/coden.html; if the abbreviation is not in this list, you may use the full journal title. (#) Author 1; Author 2; etc Journal abbreviation, year, volume, page(s). (1) Pauson, P. L. J. Organomet. Chem. 2001, 367, 3-6. (2) Kealy, T. J.; Pauson, P. L. Nature 1951, 168, 1039. (3) Woodward, R. B.; Rosenblum, M.; Whiting, M. C. J. Am. Chem. Soc. 1952, 74, 3458-3459. If your article came from an online journal, include the URL and date accessed. (#) Author 1; Author 2; etc. Journal abbreviation [online], year, volume, page. URL (accessed Month Day, Year) (4) Abd-El-Aziz, A.; Todd, E.; Shipman, P. Polymer News 2005, 30, 202-212. http://www.ingentaconnect.com/content/tandf/gpln/2005/00000030/00000007/art00001 (accessed October 20, 2008) Books without editors Books without editors are referenced by listing the authors (the last name, followed by initials), the title of the book (italics), the edition (if other than the first), the publisher (followed with a colon), place of publication, year of the publication, volume number (if any), page or pages you got the information from. Recall, if a piece of information such as the volume number or edition number is not available, you do not include it. (#) Author 1; Author 2; etc. Book title, Edition number; Publisher: Place of publication, Year; Volume number, page(s). (5) Venkataram, K. The Chemistry of Synthetic Dyes, Academic Press: New York, 1952; Vol. 1, pp 210-211. Note: This was a first edition so the edition number was not included. If your book is a later edition, make sure to include the edition number. If it is an online book include the URL and date accessed. (#) Author 1; Author 2; etc. Book title [Online], Edition number; Publisher: Place of publication, Year; Volume number, page(s). URL (accessed Month Day, Year). (6) Kuehni, R.G. Color: An Introduction to Practice and Principles [Online], 2nd Ed.; John Wiley & Sons, Inc: Hoboken, NJ, 2005, 15. http://www3.interscience.wiley.com/cgibin/bookhome/109800345 (October 20, 2008). Note: this book does not have multiple volumes so the volume number was not included. 154 Books with editors If a book has editors, it means that the different authors wrote different parts or chapters in the book. You need only mention the authors for the section you are citing. Books with editors are referenced by listing the authors (the last name, followed by initials), the chapter title (if only one chapter was relevant), the title of the book (italics), the edition (if other than the first), list the editors (last name first followed by initials), the publisher (followed with a colon), place of publication, year of the publication, volume number (if any), page or pages you got the information from. #) Author 1; Author 2; etc. Chapter title. In Book title, Edition number; Editor 1, Editor 2, etc., Eds.; Publisher: Place of publication, Year; Volume number, page(s). (7) Dodd, J.S.; Solla, L.; Berard, P.M. References. In The ACS Style Guide, 3rd Ed.; Coghill, A.M., Garson, A.R., Eds.; Oxford University Press, 2006, pp 287-341. Note: This book does not have multiple volumes, so the volume number was not included. If a book with editors is being referenced as a whole, the authors and page(s) do not need to appear. (#) Book title, Edition number; Editor 1, Editor 2, etc., Eds.; Publisher: Place of publication, Year; Volume number. (8) Acetylene Chemistry; Diederich, F., Stang, P.J., Tykwinski, R.R. Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. Note: This book was a first edition and there were no other volumes, so these sections have been omitted. If it is a book that is available online include the URL and the date the information was accessed. (#) Author 1; Author 2; etc. Chapter title. In Book title [Online], Edition number; Editor 1, Editor 2, etc., Eds.; Publisher: Place of publication, Year; Volume number, page(s). URL (accessed Month Day, Year). (9) Chauvin, R.; Lepetit, C. Theoretical Studies on Acetylenic Scaffolds. In Acetylene Chemistry [Online]; Diederich, F., Stang, P.J., Tykwinski R.R. Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp 1-50. http://www3.interscience.wiley.com/cgibin/bookhome/112098524 (accessed October 20, 2008). Note: This book was a first edition and there were no other volumes, so these sections have been omitted. Material Safety Data Sheets (MSDS) MSDSs are referenced with the substance name (italics), MSDS, manufacturing company: Location of company(city, province), Date updated. (#) Substance; MSDS, Manufacturing company: Location of company, Date updated. (10) Ferrocene; MSDS, Sigma-Aldrich: Oakville, ON, December 19, 2006. Online MSDS (#) Title; MSDS [Online]; Manufacturing company: Location of company, Date updated. URL (accessed Month Day, Year). 155 (11) Ferrocene; MSDS [Online]; Sigma-Aldrich: Oakville, ON, December 19, 2006. http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do (accessed October 20, 2008). Online Encyclopaedias Do not use Wikipedia as a source in your lab report. Marks will be deducted. Referencing software If you want to use computer software which will format your papers to any desired reference style, RefWorks is available from the UBCO library. However, it does take some time to learn how to use the RefWorks software and all references must be checked to ensure accuracy. Reference (1) Dodd, J.S.; Solla, L.; Berard, P.M. References. In The ACS Style Guide, 3rd Ed.; Coghill, A.M., Garson, A.R., Eds.; Oxford University Press, 2006, pp 287-341. 156