Predict whether the following salts will from a solution that is acidic
advertisement
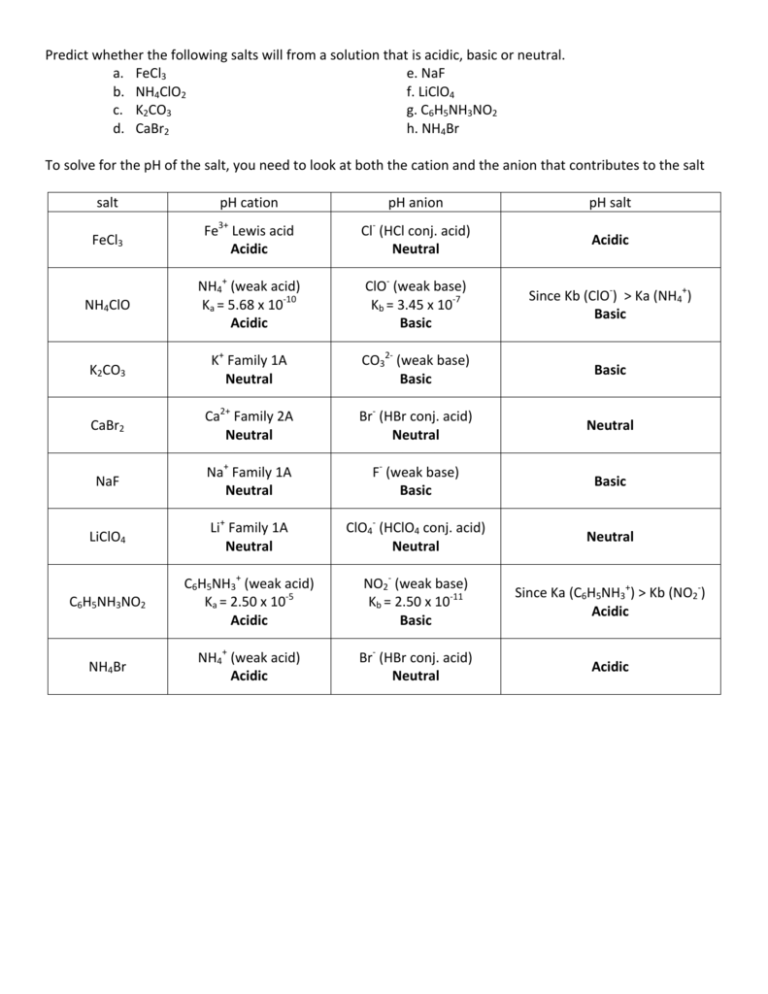
Predict whether the following salts will from a solution that is acidic, basic or neutral. a. FeCl3 e. NaF b. NH4ClO2 f. LiClO4 c. K2CO3 g. C6H5NH3NO2 d. CaBr2 h. NH4Br To solve for the pH of the salt, you need to look at both the cation and the anion that contributes to the salt salt pH cation pH anion pH salt Fe3+ Lewis acid Cl-­‐ (HCl conj. acid) FeCl3 Acidic Acidic Neutral NH4+ (weak acid) ClO-­‐ (weak base) -­‐ Since K b ( ClO ) > Ka (NH4+) NH4ClO Ka = 5.68 x 10-­‐10 Kb = 3.45 x 10-­‐7 Basic Acidic Basic K+ Family 1A CO32-­‐ (weak base) K2CO3 Basic Neutral Basic Ca2+ Family 2A Br-­‐ (HBr conj. acid) CaBr2 Neutral Neutral Neutral Na+ Family 1A F-­‐ (weak base) NaF Basic Neutral Basic Li+ Family 1A ClO4-­‐ (HClO4 conj. acid) LiClO4 Neutral Neutral Neutral C6H5NH3+ (weak acid) NO2-­‐ (weak base) Since Ka (C6H5NH3+) > Kb (NO2-­‐) C6H5NH3NO2 Ka = 2.50 x 10-­‐5 Kb = 2.50 x 10-­‐11 Acidic Acidic Basic NH4+ (weak acid) Br-­‐ (HBr conj. acid) NH4Br Acidic Acidic Neutral Worksheet #6 Chem 106-Fall 2011 1. Which of the following can form a buffer? a. HCl and CH3COOH b. H2CO3 and NaHCO3 c. NaOH and NaCl d. NaCH3COCOO and CH3COCOOH e. HClO and KClO Sections 16.1-16.4 One of the Following can form a Buffer: weak acid and its conjugate base weak base and its conjugate acid strong acid with a weak base o strong acid will react with the weak base producing weak acid strong base with a weak acid o strong base will react with the weak acid producing weak base a. strong acid/weak acid will NOT form a buffer c. strong base/neutral salt will NOT form a buffer e. weak acid-conjugate base WILL FORM a BUFFER b. weak acid-conjugate base WILL FORM a BUFFER d. weak base-conjugate acid WILL FORM a BUFFER 2. Calculate the pH of an aqueous buffer solution made from 0.150M NH4Cl and 0.100MNH3. Ka for NH4+ is 5.61x10-10 3. Calculate the pH of a buffer solution made by adding 25.5g of NaCH3CO2 (MW:82.034g/mol), in a sufficient volume of 0.550M HCH3CO2 to make 500mL of the buffer. Ka=1.8x10-5 4. What mass of NaCH3CO2 (MW:82.034g/mol) must be dissolved in 0.300L of 0.25M HCH3CO2 to produce a solution with pH=5.09? Ka= 1.8x10-5 . Assume the volume remains constant 5. Which of the following buffer systems would be the best choice to create a buffer with pH of 7.20? a. HC2H3O2/NaC2H3O2 ((Ka HC2H3O2=1.8x10-5) b. NH3/NH4Cl (Kb NH3=1.76x10-5) c. HClO2/KClO2 (Ka HClO2=1.1x10-2) d. HClO/KClO (Ka HClO=2.9x10-8) 6. What is the pH of 250.0mL of a buffer solution that is 0.0955M HCH3CO2 and 0.125M NaCH3CO2. Ka=1.8x10-5. What is the pH of the system if 20.0mL of 0.455M HCl (aq) is added?. What is the pH of the system if instead, 20.0mL of 0.455M Ca(OH)2 (aq) is added? (this are 3 separate questions) 7. Read section 16.3 of your book on buffer effectiveness and buffer capacity!!!! Elsa’s Notes: A. In order to have a buffer, we must have a weak acid and its conjugated base or a weak base and its conjugated acid present in the solution we can also form a buffer if we have a weak acid in the presence of limited amounts of a strong base o a strong base will completely react with the weak acid, forming conjugated base (since now you will have both weak acid and its conjugated base you will have a buffer) o we can also form a buffer if we have a weak base in the presence of limited amounts of a strong acid o a strong acid will completely react with the weak base, forming conjugated acid (since now you will have both weak acid and its conjugated base you will have a buffer) o B. Other 3 things to look at to know whether you have a good buffer is: ( ) ratio: ( o ) A buffer is more effective the closer the pH is to the pKa of the acid The most effective is when the ratio of ( How? )=1, because here pH=pKa ( ) ( ) Moles of conjugated acid and conjugated base: o The more moles of conjugated acid and base present, the better the buffer This is because it can tolerate bigger amounts of strong acid or strong base added to the buffer system without destructing the buffer capacity!!! Buffer capacity: o A buffer is a buffer if: pH of the buffer is within pKa + 1 or the ratio ( How? ) is higher than or equal 0.10 lower than or equal to 10 ( ( ) or ) ( ) So…. pKa + 1 o If either the ratio ( ) is outside the 0.10-10 range (lower than 0.10 or higher than 10… or pKa + 1) You no longer have a buffer!!!!







