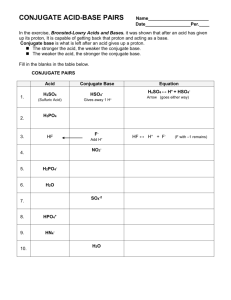
4.4. Bronsted-Lowry Theory of A&B acid : a substance which base
advertisement

Chemistry 12 Acids & Bases Unit Notes 4.4. Bronsted-Lowry Theory of A&B acid : a substance which ___________________________ base : a substance which ___________________________ H+ Typical Bronsted-Lowry A-B rxn eqn: eg1) NH3 + base H2O ⇔ acid eg2) CH3COOH ______ + NH4+ + CH3COO- H2O ⇔ _____ OHH3O+ + H2O an acid and a base????? H2O is said to be amphiprotic Amphiprotic substance: a substance which can _______________________________________ • If a substance acts as an acid around a stronger base, or a base around a stronger acid, it is an AMPHIPROTIC substance. (eg. H2O Note: every Bronsted-Lowry rxn there is an acid and a base on both sides of the rxn eqn N2H5+ A + S2B ⇔ N2H4 B* HSA* + • Acids classified as: releases one proton. Monoprotic releases two protons. Diprotic releases three protons. Triprotic releases many protons Polyprotic B* & A* are acids and bases for the reverse reaction • Bases classified as: accepts one proton. Monobasic accepts two protons. Dibasic accepts three protons. Tribasic accepts many protons Polybasic Polyprotic acids release H+ ions ____ ___ __ ______: step 1 H3PO4 step 2 H2PO4- step 3 HPO42- PO43- If a substance has a ______________ charge and still has an easily removable ______________ then the substance is ________________ Also, _______________ acids which have lost one proton are amphiprotic Hebden #11-14 - 2- H2S ↔ HS ↔ S 1 Chemistry 12 Acids & Bases Unit Notes 4.5 Conjugate Acids and Bases Conjugate acid-base pair: the pair of molecules in a rxn eqn that _________________________. Conjugate acid: _________ the extra proton Conjugate base: _________ the extra proton Bronsted-Lowry eqns have two conjugate A-B pairs: eg1) base2 acid2 ____________ | | HF + H2O ⇔ F + H3O+ |____________| acid1 base1 Conjugate Pair eg1) HF and FH3O+ and H2O Conjugate A Conjugate B What is the conjugate B for the following compounds? NH4+ HCO3- H2PO4- What is the conjugate A for the following compounds? CH3COO- HC2O4- OH- Connect and label the conjugate pairs: a) HCl(aq) + H2O(l) → H3O+ (aq) + Cl- (aq) b) H2SO4(aq) + H2O(l) → H3O+ (aq) + HSO4- (aq) Hebden #16, 19 Summary for a Bronsted-Lowry A-B rxn: Conjugate Acid +Form of A + Conjugate Base form of B Conjugate Base form+of A + Conjugate Acid form of B 2 Chemistry 12 Acids & Bases Unit Notes 4.6. “Strong and Weak” Acids and Bases Recall: Concentrated = _____ molarity (M) (mol/L) and Dilute = _____ molarity BUT in chemistry… And… Strong acid ≠ _______________ acid Weak acid ≠ ____________ acid Strong Acid (SA) or Strong Base (SB): _____________ 100%: Eg: NaOH(s) Na+(aq) + OH-(aq) HCl(g) H+(aq) + Cl-(aq) Weak Acid (WA) or Weak Base (WB): ______ than 100% dissociated: Eg: NH3(aq) + H2O(l) ⇔ NH4+(aq) + OH-(aq) HF(aq) + H2O(l) ⇔ H3O+(aq) + F-(aq) not complete dissociation ∴ eqb arrows Characteristics of Acids and Bases: are STRONG if they: dissociate __________________ in water have an undefined, or very _______ Ka value have ______________ dissociation rxn (→) are WEAK if they: do _______ dissociate completely in water have a ____________ Ka value have an ___________ dissociation rxn (⇔) Examples of STRONG Acids (p334): _____________________________________________________ Examples of STRONG Bases (p. 122): ____________________________________________________ PRACTICE: Describe the acid below as: a)monoprotic, b) diprotic, c) triprotic H2SO4(aq) → H+ (aq) + HSO4- (aq) d) strong, _________ e) weak metal hydroxides HSO4- (aq) ⇔ H+ (aq) + SO42-(aq) _________ Which is a stronger acid? HNO2 _ HPO42- Which is a stronger base? HC2O4- _ CN3 Chemistry 12 Acids & Bases Unit Notes 6. The Levelling Effect Strong acids are 100% dissociated in soln. are all equiv. to H3O+ solns of same conc. ∴ eg) 1M HI produces 1M H3O+ and 1M I1M HClO4 produces 1M H3O+ and 1M ClO4- Strengths are “leveled” Similarly, the strong bases are 100% dissociated in soln. Therefore, are all equiv. to OH- solns of that conc eg) 1M KOH produces 1M OH- and 1M K+ 1M NaOH produces 1M OH- and 1M Na+ Strengths are “leveled” Weaker A&B dissociate to different extents, so have unique strengths. Hebden # 21-27 4 Chemistry 12 Acids & Bases Unit Notes 4.4. Bronsted-Lowry Theory of A&B acid : a substance which donates a proton base : a substance which accepts a proton H+ Typical Bronsted-Lowry A-B rxn eqn: eg1) NH3 + base H2O ⇔ acid eg2) CH3COOH acid + NH4+ + CH3COO- H2O ⇔ base OHH3O+ + H2O an acid and a base????? H2O is said to be amphiprotic Amphiprotic substance: a substance which can act as either an acid or a base • If a substance acts as an acid around a stronger base, or a base around a stronger acid, it is an AMPHIPROTIC substance. (eg. H2O Note: every Bronsted-Lowry rxn there is an acid and a base on both sides of the rxn eqn N2H5+ A + S2B ⇔ N2H4 B* HSA* + • Acids classified as: releases one proton. Monoprotic releases two protons. Diprotic releases three protons. Triprotic releases many protons Polyprotic B* & A* are acids and bases for the reverse reaction • Bases classified as: accepts one proton. Monobasic accepts two protons. Dibasic accepts three protons. Tribasic accepts many protons Polybasic Polyprotic acids release H+ ions one at a time: step 1 H3PO4 step 2 H2PO4- step 3 HPO42- PO43- If a substance has a negative charge and still has an easily removable hydrogen then the substance is amphiprotic Also, polyprotic acids which have lost one proton are amphiprotic Hebden #11-14 - 2- H2S ↔ HS ↔ S 5 Chemistry 12 Acids & Bases Unit Notes 4.5 Conjugate Acids and Bases Conjugate acid-base pair: the pair of molecules in a rxn eqn that differ by one proton. Conjugate acid: has the extra proton Conjugate base: lacks the extra proton Bronsted-Lowry eqns have two conjugate A-B pairs: eg1) base2 acid2 ____________ | | HF + H2O ⇔ F + H3O+ |____________| acid1 base1 Conjugate Pair eg1) HF and FH3O+ and H2O Conjugate A HF H3O+ Conjugate B FH2O What is the conjugate B for the following compounds? NH4+ HCO3- NH3 CO32- H2PO4- HPO42- HC2O4- H2C2O4 What is the conjugate A for the following compounds? CH3COO- OH- CH3COOH H2O Connect and label the conjugate pairs: a) HCl(aq) + H2O(l) → H3O+ (aq) + Cl- (aq) c) H2SO4(aq) + H2O(l) → H3O+ (aq) + HSO4- (aq) Hebden #16, 19 Summary for a Bronsted-Lowry A-B rxn: Conjugate Acid +Form of A + Conjugate Base form of B Conjugate Base form+of A + Conjugate Acid form of B 6 Chemistry 12 Acids & Bases Unit Notes 4.6. “Strong and Weak” Acids and Bases Recall: concentrated = high molarity (M) (mol/L) and Dilute = low molarity BUT in chemistry… And… Strong acid ≠ concentrated acid Weak acid ≠ dilute acid Strong Acid (SA) or Strong Base (SB): dissociate 100%: Eg: NaOH(s) Na+(aq) + OH-(aq) HCl(g) H+(aq) + Cl-(aq) Weak Acid (WA) or Weak Base (WB): less than 100% dissociated: Eg: NH3(aq) + H2O(l) ⇔ NH4+(aq) + OH-(aq) HF(aq) + H2O(l) ⇔ H3O+(aq) + F-(aq) not complete dissociation ∴ eqb arrows Characteristics of Acids and Bases: are STRONG if they: dissociate completely in water have an undefined, or very large Ka value have complete dissociation rxn (→) are WEAK if they: do NOT dissociate completely in water have a defined Ka value have an equilib dissociation rxn (⇔) Examples of STRONG Acids (p334): HClO4, HI, HBr, HCl, HNO3, H2SO4 Examples of STRONG Bases (p. 122): NaOH, KOH, Mg(OH) 2,Ca(OH) 2,Fe(OH)3, Zn(OH)2 PRACTICE: Describe the acid below as: a)monoprotic, b) diprotic, c) triprotic H2SO4(aq) → H+ (aq) + HSO4- (aq) b&d HSO4- (aq) ⇔ H+ (aq) + SO42-(aq) a&e metal d) strong, e) weak hydroxides Which is a stronger acid? HNO2 > HPO42- Which is a stronger base? HC2O4- < CN7 Chemistry 12 Acids & Bases Unit Notes 6. The Levelling Effect Strong acids are 100% dissociated in soln. are all equiv. to H3O+ solns of same conc. ∴ eg) 1M HI produces 1M H3O+ and 1M I1M HClO4 produces 1M H3O+ and 1M ClO4- Strengths are “leveled” Similarly, the strong bases are 100% dissociated in soln. Therefore, are all equiv. to OH- solns of that conc eg) 1M KOH produces 1M OH- and 1M K+ 1M NaOH produces 1M OH- and 1M Na+ Strengths are “leveled” Weaker A&B dissociate to different extents, so have unique strengths. Hebden # 21-27 8