Imaging in the Assessment and Management of Achilles
advertisement

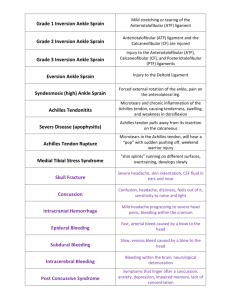
Imaging in the Assessment and Management of Achilles Tendinopathy and Paratendinitis Nevin T. Wijesekera, B.Sc., M.B.B.S., M.R.C.P., F.R.C.R.,1 James D. Calder, M.D., F.R.C.S., F.F.S.E.M.,2 and Justin C. Lee, B.Sc., M.B.B.S., M.R.C.S., F.R.C.R.1 ABSTRACT Downloaded by: NYU. Copyrighted material. Achilles tendinopathy is a common overuse injury in patients engaged in athletic activities. Tendon degeneration is often accompanied by paratendinitis. Radiologists are frequently asked to use imaging techniques to evaluate patients with problems at or around the Achilles tendon. The main imaging modalities used in the assessment of Achilles tendon disorders are plain radiography, ultrasound, and magnetic resonance imaging. In recent years, ultrasound has also been used to guide minimally invasive local treatments for Achilles tendinopathy, which may prevent the need for surgery if conservative treatments have failed. In this article, we review the imaging features of Achilles tendinopathy and consider the relative strengths and weaknesses of the various imaging techniques. The role of imaging in directing patient management is also discussed, with particular focus on ultrasound-guided treatments. KEYWORDS: Achilles, tendinopathy, paratendinitis, ultrasound, MRI, treatment A chilles tendon problems are a frequent cause of foot and ankle pain. The incidence of Achilles tendinopathy is highest in athletes, but it is also common among so-called weekend warriors in the general population. Injuries of the Achilles tendon are classified by anatomical location, occurring at either the main body of the tendon or the osseotendinous junction (Table 1). Of the Achilles overuse injuries, noninsertional Achilles tendon pathology (tendinopathy and paratendinitis) are the most common clinical diagnoses (50 to 75%), followed by insertional disorders (insertional tendinosis and retrocalcaneal bursitis) (20 to 25%).1,2 A third area involves injuries to the myotendinous junction, although these are far less frequent. The terminology used to describe Achilles tendon disorders has been confusing and often does not reflect the underlying pathology, with terms such as tendonitis, tendinosis, tendinopathy, and partial tear used for apparently similar conditions.3 In recent years, efforts to clarify the nomenclature has led to the term tendinopathy being recommended to refer to the clinical syndrome of activityrelated pain, swelling, and impaired performance.4 This clinical diagnosis includes the histological diagnoses tendinosis and paratendinitis. The term tendinosis is considered appropriate because it recognizes the degenerative changes present within the tendon substance, but the term tendonitis has fallen out of favor because there is no inflammatory response in a chronic tendinopathy. 1 nosis and Management; Guest Editors, Jeremiah C. Healy, F.R.C.P., F.R.C.R., F.F.S.E.M. and Justin C. Lee, M.R.C.S., F.R.C.R. Semin Musculoskelet Radiol 2011;15:89–100. Copyright # 2011 Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. Tel: + 1(212) 584-4662. DOI: http://dx.doi.org/10.1055/s-0031-1271961. ISSN 1089-7860. Department of Radiology, Chelsea and Westminster Hospital, London, United Kingdom; 2Department of Orthopaedic Surgery, Basingstoke and North Hampshire Hospital, Basingstoke, United Kingdom. Address for correspondence and reprint requests: Justin C. Lee, M.R.C.S., F.R.C.R., Department of Radiology, Chelsea and Westminster Hospital, 369 Fulham Road, London SW10 9NH, UK (e-mail: Justin.Lee@chelwest.nhs.uk). Sports Injury of the Lower Extremity: Role of Imaging in Diag- 89 SEMINARS IN MUSCULOSKELETAL RADIOLOGY/VOLUME 15, NUMBER 1 2011 Table 1 Classification of Achilles Tendon Injuries by Anatomical Area NONINSERTIONAL AREA Noninsertional Achilles tendinosis Achilles paratendinitis Adhesive tendinopathy Achilles tendon rupture INSERTIONAL AREA Insertional Achilles tendinosis Retrocalcaneal bursitis Retro-Achilles bursitis Distal Achilles tendo-fasciitis Avulsion fracture of the calcaneus No specific temporal criteria define tendon overuse injuries as acute or chronic. El Hawary et al suggested that the Achilles tendon disorder should be classified as ‘‘acute’’ if symptoms are present for < 2 weeks, ‘‘subacute’’ is present for 2 to 6 weeks, and ‘‘chronic’’ if present for > 6 weeks.5 These arbitrary distinctions are not based on clinical criteria or histopathological findings but provide a useful framework for the condition. Clinical differentiation between the many causes of achillodynia can be difficult, and lesions may coexist. However, it is important for clinicians to distinguish between the various types of Achilles tendon disorders because this will affect treatment and rehabilitation. The goal of management is to return patients to their desired level of activity without significant residual pain. Achieving an accurate diagnosis requires a detailed injury history, a comprehensive knowledge of local anatomy, and, often, diagnostic imaging techniques.6 This article reviews the imaging features of Achilles tendinopathy and outlines the potential uses and limitations of various imaging modalities in the noninvasive evaluation of the tendon and the role of imaging in guiding treatment. ANATOMY The Achilles tendon is the conjoint aponeurosis of the gastrocnemius-soleus musculotendinous unit (Fig. 1). The medial and lateral heads of the gastrocnemius muscle originate from the posterior surface of the femoral condyles. The soleus originates from the posterior surfaces of the tibia, fibula, and interosseous membrane. The muscles that form the Achilles tendon act mainly to plantarflex the ankle, and the gastrocnemius muscle also flexes the knee joint. Together with the plantaris tendon and the retrocalcaneal (Kager’s) fat pad, the Achilles tendon fills the distal part of the posterior compartment of the calf. The Achilles tendon inserts on the posterior surface of the calcaneus distal to the posterior-superior calcaneal tuberosity. Approximately 12 to 15 cm prox- Figure 1 Sagittal T2-weighted magnetic resonance image showing normal Achilles tendon (arrows). imal to its insertion, the fibers of the conjoint tendon begin to spiral through 90 degrees, such that the medial fibers proximally become the most posterior fibers distally at the insertion. This rotation produces stress within the tendon, shown to be greatest 2 to 6 cm above the calcaneal insertion, which is a common site for tendinopathy. The plantaris tendon runs along the medial aspect of the Achilles tendon (Fig. 2). It may become confluent with the Achilles tendon or Figure 2 Axial T2-weighted MR image demonstrating plantaris tendon (curved arrow) on the medial aspect of the Achilles tendon (straight arrow). Downloaded by: NYU. Copyrighted material. 90 IMAGING OF ACHILLES TENDINOPATHY AND PARATENDINITIS/WIJESEKERA ET AL 91 Figure 3 Extended field-of-view sonogram of the posterior calf. Note the anatomical variant of an accessory (Acc) soleus muscle running alongside the flexor hallucis longus muscle (FHL). have an independent insertion on the calcaneus. Although often thought of as a vestigial structure— the plantaris is absent in 7 to 20% of limbs—pathology of the plantaris tendon is an important differential diagnosis for pain arising from the proximal posterior aspect of the leg.7,8 An accessory soleus muscle is a rare anatomical variant that may insert directly onto the anterior margin of the Achilles tendon or via a separate tendon on the calcaneus, anteromedial to the Achilles tendon (Fig. 3). When an accessory soleus muscle is present, it may manifest as a soft tissue mass bulging medially between the distal tibia and the Achilles tendon.9 Two important bursal sacs are associated with the Achilles tendon insertion: a retrocalcaneal bursa between the insertion of the tendon and the calcaneus, and a subcutaneous bursa between the distal tendon and the skin.10 Pathology in the Achilles tendon can cause these bursae to become inflamed. The retrocalcaneal bursa may be seen in normality, although a bursa > 1 mm anteroposteriorly, 11 mm transversely, or 7 mm craniocaudally is abnormal.11 The subcutaneous bursa is acquired, and its presence usually indicates local trauma or inflammation. The Achilles tendon is covered along its entire length by a thin paratenon sheath. The paratenon is composed of both visceral and parietal layers, rather than a true synovial sheath, and facilitates gliding movement of the tendon within the surrounding tissues. The paratenon forms a thin space between the tendon and the crural fascia. Under the paratenon lies the epitenon, which is a loose connective tissue layer surrounding the Achilles tendon. The inner surface of the epitenon is continuous with the endotenon, which binds the muscle fibers together and also provides the neurovascular supply to the tendon. The Achilles tendon receives its blood supply from three sources: the osseous insertion, the musculotendinous junction, and via the paratenon.12 Nerve fibers follow the vascular channels, forming a rich plexus in the paratenon before penetrating the epitenon and terminating as nerve endings on the tendon surface. Studies ETIOLOGY AND PATHOPHYSIOLOGY Achilles tendinopathy is seen in both athletic and nonathletic individuals and, although its cause remains unclear, it is strongly associated with overuse and repetitive tendon loading. Pathogenesis is considered to be multifactorial, with mechanical, vascular, neural, and ‘‘failure of healing’’ models having been proposed. Training errors are implicated in up to 70% of running injuries.15 These include running too long a distance, running at too great an intensity, or performing too much uphill or downhill training. In acute Achilles tendinopathy, an inflammatory cellular reaction occurs, with circulatory impairment and edema formation. If this acute condition is untreated or inadequately treated, the inflammatory exudate may organize and form peritendinous adhesions.16 Tendon degeneration (tendinosis) may be found in conjunction with peritendinous adhesions, although a causal link has not been established. The chronic phase of Achilles tendinopathy is characterized by a ‘‘disordered’’ healing response, with collagen degeneration, increase in ground substance, and neovascularity. Although acute inflammatory tendonitis was once thought to precede tendinosis, inflammatory cell infiltration has not been shown in biopsy specimens of chronically painful tendons.17 The origin of pain in chronic Achilles tendinopathy is still unclear. Given the paucity of evidence supporting an inflammatory basis, alternative theories for the cause of pain have been proposed. These include increased levels of nociceptive neurotransmitters (e.g., glutamate and substance P) and peritendinous vasculoneural ingrowth.18,19 DIAGNOSIS Achilles tendinopathy is clinically manifest by pain, swelling, and impaired performance. A correct diagnosis can often be established by clinical examination alone. However, differentiating between Achilles tendon disorders can be difficult, and Achilles tendon ruptures are reportedly missed by up to 20% of primary care physicians.20 If a diagnosis is not clear, imaging may be useful to identify pathology within and around the Achilles tendon. The imaging modalities most commonly used in the evaluation of the Achilles tendon are conventional radiography, ultrasonography, and magnetic resonance imaging (MRI). Downloaded by: NYU. Copyrighted material. have demonstrated a ‘‘watershed area’’ of relative hypovascularity 2 to 6 cm proximal to the tendon insertion, corresponding with the site of pathology in noninsertional tendinopathy.13,14 The poor blood supply to this region is implicated in the pathophysiology of tendinopathy and tendon tears. SEMINARS IN MUSCULOSKELETAL RADIOLOGY/VOLUME 15, NUMBER 1 Plain Radiography Plain radiographs provide limited information on soft tissues structures due to lack of soft tissue contrast and therefore not recommended routinely for all patients with suspected Achilles tendinopathy. However, conventional radiography is easily accessible and inexpensive, and it may provide useful information in some patients with posterior heel pain. Plain radiographs should include a lateral weightbearing view of the foot and ankle and an axial view of the heel. On conventional radiography, the Achilles tendon has well-defined margins, particularly anteriorly, due to the interface between the anterior surface of the tendon and the pre-Achilles (Kager’s) fat pad. This sharp interface may be obscured in patients with Achilles tendinopathy.6 Plain radiography can also demonstrate calcification or ossification within the Achilles tendon, which can be a feature of chronic tendinosis or previous tendon rupture. In patients with insertional tendinopathy, there is often a posterosuperior exostosis arising from the lateral side of the calcaneal tuberosity, known as Haglund’s deformity. Also known as a ‘‘pump bump’’ due to the association with certain shoes, Haglund’s deformity is seen in up to 60% of patients with insertional Achilles tendinopathy, although not all patients with pump bumps have insertional tendinopathy.21 In 1982, Pavlov and colleagues defined the radiological criteria of a Haglund’s deformity by the use of parallel pitch lines on a lateral radiograph of the heel (Fig. 4).22 Figure 4 Lateral radiograph of the ankle demonstrating the parallel pitch line method for diagnosing Haglund’s deformity. Line A is drawn from the anterior tubercle to the medial tubercle. Line B parallels line A and starts at the highest point of the posterior facet of the subtalar joint surface. The tip of the posterior superior tubercle of the calcaneum (arrow) lies above line B, diagnostic of a Haglund’s deformity. 2011 Ultrasound Ultrasound examination plays an important role in the evaluation of musculoskeletal disorders owing to its low cost, relative availability, noninvasiveness, and dynamic character. The technique also allows easy contralateral comparison.6 Furthermore, ultrasound is increasingly being used to direct therapeutic interventions for Achilles tendinopathy refractory to conservative treatment regimes, thus offering an alternative to surgical management. Ultrasonography carries a high positive predictive value for Achilles tendinopathy, although a negative examination can occur in patients with clinically proven tendinopathy.23 The Achilles tendon is ideally examined with the patient lying prone with the foot hanging freely. A linear or small footprint high frequency (7 to 12 MHz) ultrasound probe should be used. Ultrasonographic evaluation of the tendon is ideally performed in both transverse and longitudinal planes. The ultrasound probe should be aligned perpendicular to the tendon fibers to avoid artifacts from acoustic anisotropy, which can simulate the appearance of tendinopathy. Simultaneous use of color or power Doppler ultrasound can add information about blood flow. Extended-field-of-view scanning enables panoramic imaging of the Achilles tendon from its origin to the calcaneus.24 The healthy Achilles tendon exhibits an echogenic pattern of parallel fibrillar lines in the longitudinal plane and an echogenic round-to-ovoid shape in the transverse plane. Normal tendon thickness ranges between 4 mm and 7 mm.25 The retrocalcaneal and subcutaneous bursae, if present, are usually well defined by ultrasonography. Figure 5 A 57-year-old woman long-distance runner with chronic noninsertional Achilles tendinopathy. Transverse sonogram with color Doppler (color not shown) demonstrating neovascularity (arrow) in the tendon. Note also the reduced echogenicity in the superficial tendon (arrowhead), consistent with tendinopathy. Downloaded by: NYU. Copyrighted material. 92 IMAGING OF ACHILLES TENDINOPATHY AND PARATENDINITIS/WIJESEKERA ET AL 93 Figure 6 A 31-year-old male professional soccer player with chronic noninsertional Achilles tendinopathy. Extended field-of-view sonogram displaying the entire length of the Achilles tendon from the myotendinous junction to the calcaneal insertion. Note the fusiform thickening in the midportion of the tendon, characteristic of tendinopathy. Typical ultrasonographic findings in patients with Achilles tendinopathy include spindle shape/fusiform thickening of the tendon and ill-defined hypoechoic areas within the tendon, which may or may not have associated areas of increased vascularity on color Doppler (Figs. 5 and 6). Blood flow is not detected in the normal Achilles tendon, but color Doppler often reveals blood flow in tendinopathic tendons. This neovascularization has been linked to greater pain, poorer function, and longer symptoms in Achilles tendinopathy.26 In acute Achilles paratendinopathy, ultrasound can reveal fluid around the tendon. Peritendinous adhesions, seen as thickening of the hypoechoic paratenon, may be apparent in the chronic form of the disorder and can be defined with dynamic sonographic examination (Fig. 7).27 Sonoelastography is a new ultrasound-based technique that is able to evaluate tissue elasticity. Real-time sonoelastography has been shown to perform well compared with conventional ultrasound findings in depicting the Achilles tendons of healthy volunteers.28 Painful thickened Achilles tendons show increased stiffness on Figure 7 A 35-year-old male rugby union player with Achilles paratendinitis. Transverse sonogram showing thickening of the hypoechoic paratenon (between arrowheads). Magnetic Resonance Imaging Owing to its multiplanar imaging capabilities and excellent soft tissue contrast characteristics, MRI is a useful modality for imaging the Achilles tendon. However, MRI is expensive and may not be widely available in some countries, and scanning can be time consuming. As with ultrasound, MRI has a high sensitivity and specificity for detection of abnormalities in cases of achillodynia, but MRI has a greater correlation with 12-month clinical outcome.30 Several MRI scanners and pulse sequences are now available. The choice of sequences varies between institutions, but conventional imaging of the Achilles tendon should include a combination of T1-weighted (T1W) and fluid-sensitive sequences in the axial and sagittal planes.31 Our routine ankle MRI protocol includes fat-saturated T1W sequences in both planes, axial fat-saturated protondensity, and sagittal short tau inversion recovery (STIR) imaging. Some authors favor fat-suppressed T2-weighted (T2W) fast spin-echo sequences. In addition, we also advocate the routine use of intravenous gadolinium enhanced fat-saturated T1W imaging. Contrast enhancement within the tendon suggests neovascularity, whereas Figure 8 A 54-year-old male cricketer with chronic Achilles tendinopathy. Sagittal short tau inversion recovery magnetic resonance image showing fusiform swelling within the midportion of the Achilles tendon (arrows) and focal areas of intratendinous high signal. Downloaded by: NYU. Copyrighted material. sonoelastography compared with normal tendons.29 However, further studies are required to define the role of real-time sonoelastography in the routine assessment of Achilles tendinopathy. SEMINARS IN MUSCULOSKELETAL RADIOLOGY/VOLUME 15, NUMBER 1 Figure 9 A 34-year-old male long-distance runner with posterior heel pain. Sagittal short tau inversion recovery magnetic resonance image demonstrating insertional Achilles tendinopathy (arrow) in association with the Haglund’s deformity (H). paratendinous enhancement is indicative of paratendinitis. Importantly, contrast-enhanced MRI sequences can aid differentiation of paratendinitis from effusion of bursitis, which directs management decisions. MRI of the Achilles tendon using ultrashort TE pulse sequences has recently been promoted because it has been shown to provide anatomical detail not apparent with conventional sequences and increased conspicuity of contrast enhancement in tendinopathy.32 The normal Achilles tendon is of low signal on conventional MRI pulse sequences, reflecting the compact ultrastructural arrangement and low intrinsic water content. The MRI findings in Achilles tendinopathy include anteroposterior tendon thickening, fusiform tendon shape, and areas of high signal within the tendon on T1W, T2W, and STIR sequences (Figs. 8 and 9).33 Areas of intratendinous increased signal are thought to represent areas of collagen disruption and partial tearing. It should be noted that intratendinous high signal can also be found in asymptomatic individuals that may reflect subclinical tendinopathy or mucoid degeneration. Artifact related to the magic angle phenomenon can also cause areas of increased signal within normal tendons, particularly on T1W sequences that have a low TE. This effect is observed when the orientation of tendon fibers relative to the static magnetic field approaches 55 degrees, which can occur as the Achilles tendon fibers spiral internally. The magic angle phenomenon can be deliberately exploited when imaging the Achilles tendon. By imaging the Achilles tendon at the magic angle (the long 2011 Figure 10 A 44-year-old male runner with Achilles paratendinitis. Axial T2-weighted magnetic resonance image showing a rim of paratendinous fluid (between arrowheads) around the anterior and lateral aspects of the Achilles tendon. axis of the tendon at 55 degrees to the main magnetic field, rather than the normal 0 degrees8), pathological intratendinous signal change on STIR and contrastenhanced sequences becomes more apparent.34,35 Achilles paratendinopathy may be seen on T2W or STIR sequences as linear or reticular high signal areas alongside the deep surface of the tendon, representing areas of edema or increased vascularity (Fig. 10).27 Figure 11 A 35-year-old elite triple jumper with a long history of posterior calf pain. Sagittal short tau inversion recovery magnetic resonance image demonstrating chronic plantaris tendon rupture. There is marked inflammation (arrowheads) around the retracted end of the ruptured tendon (arrow). Downloaded by: NYU. Copyrighted material. 94 MRI is also useful to differentiate Achilles tendinopathy from other causes of calf pain. Plantaris tendon strains or rupture, for example, are commonly misdiagnosed as injury to the Achilles tendon. Plantaris strain may be seen on MRI as abnormally high signal intensity both within and adjacent to the fibers of the plantaris muscle on T2W or STIR sequences. Rupture of the plantaris most often occurs at the myotendinous junction, resulting in proximal retraction of the muscle (Fig. 11). Complete plantaris rupture may appear as a high signal intensity mass located between the soleus muscle and the medial head of the gastrocnemius on T2W and fat-suppressed images. TREATMENT The traditional approach to managing chronic Achilles tendinopathy is aimed at symptom control, with therapeutic options falling into either conservative or operative groups.14,36 Conservative measures are usually attempted in combination for between 3 to 6 months but may be unsuccessful in up to 25% of patients. Most authors report up to 85% success rates from open surgery; however this may be lower in nonspecialized clinical practices.37 Therefore, it is usual to exhaust all nonoperative measures before considering surgery. Recently, several minimally invasive treatments have been used with the aim of promoting healing. Imaging is useful to guide these interventions, ensuring that treatment is delivered to the exact site of pathology (Fig. 12). Conservative Treatments Many conservative treatments are available to patients with painful Achilles tendons, but the evidential basis for most of these therapies remains sparse. Strategies such as rest (complete or modified activity), orthotic treatments, pharmacological agents, and various physical therapy modalities (heat, ultrasound, and electrical stimulation), are used in combination with the aim of correcting possible etiological factors and controlling symptoms. Treatments that have been investigated with randomized controlled trials include eccentric musculoskeletal training, topical glyceryl trinitrate (GTN), nonsteroidal anti-inflammatory drugs, and extracorporeal shock wave therapy.14,38 A regimen of intensive heavy-load eccentric muscle training has been shown to be superior to concentric training for the treatment of chronic Achilles tendinopathy. Alfredson and colleagues38 developed a model of eccentric training in which the load is increased until the patient experiences pain. This 12-week program is successful in 90% of patient with midportion Achilles tendon pain, but only 30% of those with insertional pain.39,40 Topical GTN, a prodrug of nitric oxide, is thought to induce tendon healing by stimulating collagen synthesis in fibroblasts. A double-blind randomized study involving 65 patients with chronic Achilles tendinopathy compared the continuous application of a GTN patch to the tendon with a placebo patch. At 6 months, activity pain in the treatment group was significantly reduced compared with the placebo group.41 Nonsteroidal anti-inflammatory drugs (NSAIDs) are often used in the management of acute athletic injuries for analgesic purposes. However, there is evidence that NSAIDs have little or no effect on the clinical outcome of chronic Achilles tendinopathy and may even impair tendon healing by inhibiting tendon cell migration and proliferation.42,43 Extracorporeal shock wave therapy (ESWT) is a treatment in which acoustic shock waves are directed through the skin to the affected area. Ultrasound guidance can be used to optimally position the device. The mechanism of action is unknown, but it is speculated that the energy delivered may promote diffusion of cytokines across vessel walls, resulting in the stimulation of a healing cascade. A randomized controlled trial of 75 patients compared ESWT, eccentric loading, and a waitand-see policy for the treatment of chronic midportion Achilles tendinopathy. At 4-month follow-up, there was no difference in clinical outcome between those treated by EWST and those treated by eccentric loading. However, both of these therapies were significantly better than the wait-and-see policy.44 Minimally Invasive Treatments Figure 12 Transverse sonogram showing a needle (arrows) within the Achilles tendon (AT) during a dry needling procedure. LOCAL ANTI-INFLAMMATORY INJECTIONS Local corticosteroid injections are commonly used in the treatment of tendinopathies. However, the role of 95 Downloaded by: NYU. Copyrighted material. IMAGING OF ACHILLES TENDINOPATHY AND PARATENDINITIS/WIJESEKERA ET AL SEMINARS IN MUSCULOSKELETAL RADIOLOGY/VOLUME 15, NUMBER 1 2011 Figure 13 Longitudinal sonograms with color Doppler (color not shown) of a tendinopathic Achilles tendon (AT) (A) before and (B) immediately after injection of hyperosmolar dextrose solution into the neovessels. Note the absence of color flow within the tendon following injection of the sclerosant. inflammation in chronic tendinopathy is unclear, and the rationale for the use of anti-inflammatory injections in Achilles tendinopathy is controversial. Furthermore, many clinicians advise against injection of corticosteroid in or around the Achilles tendon due to the potential risk of tendon rupture, although this has not been substantiated in large studies.45 Other risks, including fat atrophy and skin discoloration, are also well recognized. Fredberg et al46 performed a double-blind placebo-controlled study of peritendinous triamcinolone injections in 24 athletes with chronic Achilles tendonitis, in which sonography was used to diagnosis and direct placement of the injections. After 6 months, the steroid injections had a significant effect in reducing pain and thickening of tendons, compared with no change in the placebo-treated group. However, when combined with aggressive rehabilitation, many patients suffered a relapse of symptoms within 6 months. Ultrasound-guided peritendinous injections of two new anti-inflammatory drugs, adalimumab (a tumor necrosis factor a blocker) and anakinra (an interleukin-1 receptor antagonist), have also been evaluated in a small study including patients with chronic Achilles tendinopathy. Both drugs improved pain sensation at rest, although neither had a significant beneficial effect on tendon thickness.47 Achilles tendinopathy, randomized to treatment with either polidocanol (5 mg/dL) or lidocaine (5 mg/mL) plus adrenaline (5 mg/mL).48 Both polidocanol and the combination of lidocaine plus adrenaline have immediate local anesthetic and vasoconstrictive effects, but polidocanol also has a sclerosing effect. After a maximum of two injections, the short-term results (mean follow-up: 3 months) showed significantly reduced tendon pain following treatment with polidocanol but not with the nonsclerosing combination. In a pilot study, the same investigators reported good results in 8 of 11 patients with insertional tendinopathy, where neovascularization was demonstrated with Doppler ultrasound.47 Based on the same rationale as sclerotherapy, electrocoagulation has also been used to eliminate neovessels but is potentially more destructive.52 Chan et al53 hypothesized that high-volume ultrasound-guided injection of normal saline around the Achilles tendon would produce local mechanical effects causing neovessels to rupture or occlude. A local anesthetic and steroid combination was injected between the anterior aspect of Achilles tendon and Kager’s fat pad, followed by 40 mL of injectable saline (Fig. 14). They OBLITERATION OF NEOVESSELS Tendon neovascularization is a common feature on color Doppler ultrasound of painful Achilles tendons but not in those that are pain free. This observation led to the hypothesis that obliterating the new vessels may reduce refractory Achilles tendon pain (Fig. 13). Alfredson and Öhberg performed a series of studies using polidocanol as a sclerosing agent in the treatment of chronic tendinopathies.48–51 Using ultrasound and color Doppler guidance, the injections targeted areas of neovascularization just outside the ventral part of the tendon. One study involved 20 patients with chronic midportion Figure 14 Extended field-of-view sonogram following injection of 50 mL of normal saline into Kager’s fat pad for treatment of Achilles tendinopathy (AT). Note how the fluid tracks up to the musculotendinous junction (arrow). Downloaded by: NYU. Copyrighted material. 96 IMAGING OF ACHILLES TENDINOPATHY AND PARATENDINITIS/WIJESEKERA ET AL STIMULATION OF HEALING RESPONSE The pathophysiological basis of Achilles tendinopathy may be explained as a disordered wound-healing response. This model is supported by histological features of haphazard healing.54 Therapies that directly target the abnormal areas of tendon aim to interrupt the degenerative cycle by initiating a wound-healing cascade. It is hypothesized that the inflammatory response induces the formation of granulation tissue, ultimately leading to improved tendon strength and clinical outcomes. The technique known as prolotherapy, whereby small volumes of an irritant solution are injected around a painful tendon or ligament, has long been in use. The injected solution is thought to initiate a local inflammatory response, causing fibroblast proliferation and subsequent collagen production, resulting in increased tendon strength. In patients with chronic Achilles pain, Maxwell et al55 modified this technique by injecting hyperosmolar dextrose directly into areas of tendinopathy under ultrasound guidance. The study showed that in the 33 treated tendons there was a significant reduction in pain at rest and during tendon-loading activities. Dry needling is the repeated lancing of an abnormal area of tendon to incite internal hemorrhage. The consequent inflammatory response may lead to formation of granulation tissue that strengthens the tendon. The technique may be combined with intratendinous injection of autologous whole blood or autologous plateletrich plasma. These injectates are thought to provide cellular and humoral mediators to promote healing in areas of degeneration. Ultrasound-guided dry needling followed by autologous blood injections has been shown in patients with refractory epicondylitis and patellar tendinosis, but no studies of this technique have been published in patients with Achilles tendinopathy.56,57 tendon to break up adhesions. Volume adhesiotomy may be performed using ultrasound guidance to better direct the injection into the paratenon/tendon interspace. Peritendinous injection of low molecular weight heparin has also been used with the aim of limiting the formation of adhesions. However, some evidence indicates there is no beneficial effect, and it has been suggested that heparin, in itself, causes a degenerative tendinopathy.59 Other injectates such as aprotinin (a protease inhibitor) have been used in the management of Achilles tendinopathy. Brown et al60 performed a double-blind placebo-controlled trial in patients with Achilles tendinopathy, comparing aprotinin injections with saline injections (0.9%) as part of a rehabilitation program. Thirty-three affected tendons were treated with three peritendinous injections, each a week apart. In this study, aprotinin was not shown to offer any statistically significant benefit over placebo. Surgery Open procedures include paratenon stripping and debridement of tendinopathic lesions. In patients with no evidence of true tendinosis on imaging, adhesiolysis may be considered whereby adhesions between the tendon and the paratenon are divided.61,62 In patients with frank paratendinitis, the paratenon may also be excised. Success rates of 86 to 96% have been described for such treatment of isolated paratendinitis.61,63 If there are discrete tendinopathic lesions on imaging, these can be identified at surgery as macroscopically dull and yellowed areas that may contain crystalline deposits compared with the glistening white appearance of normal tendon. This abnormal tissue can be excised and the tendon repaired with resorbable sutures (Figs. 15 and 16). In those patients where there is no discrete area of abnormality but a more generalized tendinopathy is present, multiple longitudinal incisions may be made in the tendon because this has been shown to initiate PERCUTANEOUS TENOTOMY Open longitudinal tenotomy is an established surgical treatment for chronic Achilles tendinopathy if conservative measures fail. The operation aims to promote wound repair by modulation of the tendon cell-matrix environment. Testa et al58 evaluated ultrasound-guided percutaneous longitudinal tenotomy in 75 athletes with chronic achillodynia and found that their results were comparable to those reported after more extensive open surgical procedures. OTHER TREATMENTS Volume adhesiotomy, also known as brisement, is the injection under pressure of dilute local anesthetic or saline solution between the paratenon and Achilles Figure 15 Photograph of swollen Achilles tendon at open surgery. Downloaded by: NYU. Copyrighted material. found that high-volume injections significantly reduced pain and improved function in 30 patients with chronic Achilles tendinopathy. 97 SEMINARS IN MUSCULOSKELETAL RADIOLOGY/VOLUME 15, NUMBER 1 2011 problems. Steenstra et al reported on 16 patients undergoing this technique with a 2.7-mm arthroscope following a minimum period of 6 months of conservative management.73 At a follow-up of 2 to 7 years, all patients had improvement in their symptoms. Several other authors have reported similarly promising results in a small series of patients, and all report few complications.74–76 Figure 16 Photograph taken during open debridement procedure with excision of macroscopically abnormal tissue. vascular ingrowth and a healing response.62,64 Neovascularization and accompanying sensory nerves from the ventral paratendinous tissue have also been implicated in the development of pain transmission in Achilles tendinopathy.65,66 Therefore, in all surgical interventions, the anterior attached fat pad with ‘‘neovessels and/or nerves’’ should be dissected and released from the Achilles tendon. Even after extensive debridement, there is usually enough tendon to achieve side-to-side closure of the tenotomy. However, if > 50% of the Achilles tendon has been excised, it is often necessary to augment the repair using a turn-down flap of more proximal tendon or perform a tendon transfer using flexor digitorum longus, flexor hallucis longus, or even peroneal tendons.67–69 The results of surgery following the debridement of intratendinous lesions are not as good as those for paratendinitis. Nelen reported a success rate of 73% for patients undergoing open surgical debridement of tendinopathic lesions.61 Paavola demonstrated a significant difference in outcome between patients with a focal intratendinous lesion and those without in terms of success (54% and 88%, respectively).70 Maffulli et al also noted that those patients with chronic tendinopathy had a worse outcome: In 14 patients undergoing surgery with an average duration of symptoms of 87 months, only 5 had good or excellent outcomes, and 6 required further surgery.71 The complications of open procedures are well documented. In a series of 432 consecutive patients, Paavola et al presented a complication rate of 11% and a reoperation rate of 3%.72 The complications included wound edge necrosis (3%), superficial infection (2.5%) and sural nerve irritation (1%). Other complications included seroma, hematoma and fibrotic reactions, and one thrombosis. The advancement in endoscopic techniques and arthroscopic shavers has enabled the development of minimally invasive procedures to improve recovery time and reduce the incidence of wound-healing CONCLUSION Achilles tendinopathy is a common overuse injury in patients engaged in athletic activities. The imaging modalities most often used in the diagnostic assessment of the Achilles tendon include plain radiography, ultrasound, and MRI. Conservative strategies remain the mainstay of treatment. Although surgery is occasionally required, ultrasound may be used to guide local therapeutic interventions. Therefore, the radiologist plays an increasingly important role in the diagnosis and treatment of Achilles tendinopathy. REFERENCES 1. Kvist M. Achilles tendon injuries in athletes. Sports Med 1994;18(3):173–201 2. Järvinen TA, Kannus P, Paavola M, Järvinen TL, Józsa L, Järvinen M. Achilles tendon injuries. Curr Opin Rheumatol 2001;13(2):150–155 3. Werd MB. Achilles tendon sports injuries: a review of classification and treatment. J Am Podiatr Med Assoc 2007; 97(1):37–48 4. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy 1998;14(8):840–843 5. el Hawary R, Stanish WD, Curwin SL. Rehabilitation of tendon injuries in sport. Sports Med 1997;24(5):347–358 6. Bleakney RR, White LM. Imaging of the Achilles tendon. Foot Ankle Clin 2005;10(2):239–254 7. Spina AA. The plantaris muscle: anatomy, injury, imaging, and treatment. J Can Chiropr Assoc 2007;51(3):158–165 8. Simpson SL, Hertzog MS, Barja RH. The plantaris tendon graft: an ultrasound study. J Hand Surg Am 1991;16(4): 708–711 9. Cheung Y, Rosenberg ZS. MR imaging of the accessory muscles around the ankle. Magn Reson Imaging Clin N Am 2001;9(3):465–473, x 10. Bianchi S, Martinoli C, Gaignot C, De Gautard R, Meyer JM. Ultrasound of the ankle: anatomy of the tendons, bursae, and ligaments. Semin Musculoskelet Radiol 2005; 9(3):243–259 11. Bottger BA, Schweitzer ME, El-Noueam KI, Desai M. MR imaging of the normal and abnormal retrocalcaneal bursae. AJR Am J Roentgenol 1998;170(5):1239–1241 12. Carr AJ, Norris SH. The blood supply of the calcaneal tendon. J Bone Joint Surg Br 1989;71(1):100–101 13. Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK. Blood supply of the Achilles tendon. J Orthop Res 1998;16(5):591–596 Downloaded by: NYU. Copyrighted material. 98 14. Hennessy MS, Molloy AP, Sturdee SW. Noninsertional Achilles tendinopathy. Foot Ankle Clin 2007;12(4):617–641, vi–vii 15. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med 1978;6(2):40–50 16. Paavola M, Kannus P, Järvinen TA, Khan K, Józsa L, Järvinen M. Achilles tendinopathy. J Bone Joint Surg Am 2002;84-A(11):2062–2076 17. Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand 1997;68(2):170–175 18. Khan KM, Cook JL. Overuse tendon injuries: where does the pain come from? Sports Med Arthrosc Rev 2000;8:17–31 19. Öhberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc 2001;9(4):233–238 20. Bude RO, Adler RS, Bassett DR. Diagnosis of Achilles tendon xanthoma in patients with heterozygous familial hypercholesterolemia: MR vs sonography. AJR Am J Roentgenol 1994; 162(4):913–917 21. Myerson MS, McGarvey W. Disorders of the Achilles tendon insertion and Achilles tendinitis. Instr Course Lect 1999;48:211–218 22. Pavlov H, Heneghan MA, Hersh A, Goldman AB, Vigorita V. The Haglund syndrome: initial and differential diagnosis. Radiology 1982;144(1):83–88 23. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil 2008;30(20–22):1608–1615 24. Daftary A, Adler RS. Sonographic evaluation and ultrasound-guided therapy of the Achilles tendon. Ultrasound Q 2009;25(3):103–110 25. Kainberger FM, Engel A, Barton P, Huebsch P, Neuhold A, Salomonowitz E. Injury of the Achilles tendon: diagnosis with sonography. AJR Am J Roentgenol 1990;155(5):1031–1036 26. Mitchell AW, Lee JC, Healy JC. The use of ultrasound in the assessment and treatment of Achilles tendinosis. J Bone Joint Surg Br 2009;91(11):1405–1409 27. Paavola M, Järvinen TA. Paratendinopathy. Foot Ankle Clin 2005;10(2):279–292 28. Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol 2009;64(12):1196–1202 29. Sconfienza LM, Silvestri E, Cimmino MA. Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol 2010;28(3):373–378 30. Khan KM, Forster BB, Robinson J, et al. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br J Sports Med 2003;37(2):149–153 31. Schweitzer ME, Karasick D. MR imaging of disorders of the Achilles tendon. AJR Am J Roentgenol 2000;175(3): 613–625 32. Robson MD, Benjamin M, Gishen P, Bydder GM. Magnetic resonance imaging of the Achilles tendon using ultrashort TE (UTE) pulse sequences. Clin Radiol 2004;59(8):727–735 33. Karjalainen PT, Soila K, Aronen HJ, et al. MR imaging of overuse injuries of the Achilles tendon. AJR Am J Roentgenol 2000;175(1):251–260 34. Oatridge A, Herlihy A, Thomas RW, et al. Magic angle imaging of the achilles tendon in patients with chronic tendonopathy. Clin Radiol 2003;58(5):384–388 35. Marshall H, Howarth C, Larkman DJ, Herlihy AH, Oatridge A, Bydder GM. Contrast-enhanced magic-angle MR imaging of the Achilles tendon. AJR Am J Roentgenol 2002;179(1):187–192 36. Solan M, Davies M. Management of insertional tendinopathy of the Achilles tendon. Foot Ankle Clin 2007;12(4):597–615, vi 37. Paavola M, Kannus P, Orava S, Pasanen M, Järvinen M. Surgical treatment for chronic Achilles tendinopathy: a prospective seven month follow up study. Br J Sports Med 2002;36(3):178–182 38. Alfredson H, Cook J. A treatment algorithm for managing Achilles tendinopathy: new treatment options. Br J Sports Med 2007;41(4):211–216 39. Fahlström M, Jonsson P, Lorentzon R, Alfredson H. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc 2003;11(5): 327–333 40. Öhberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med 2004;38(1):8–11, discussion 11 41. Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical glyceryl trinitrate treatment of chronic noninsertional achilles tendinopathy. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 2004;86A(5):916–922 42. Aström M, Westlin N. No effect of piroxicam on achilles tendinopathy. A randomized study of 70 patients. Acta Orthop Scand 1992;63(6):631–634 43. Tsai WC, Hsu CC, Chou SW, Chung CY, Chen J, Pang JH. Effects of celecoxib on migration, proliferation and collagen expression of tendon cells. Connect Tissue Res 2007;48(1): 46–51 44. Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med 2007; 35(3):374–383 45. Speed CA. Injection therapies for soft-tissue lesions. Best Pract Res Clin Rheumatol 2007;21(2):333–347 46. Fredberg U, Bolvig L, Pfeiffer-Jensen M, Clemmensen D, Jakobsen BW, Stengaard-Pedersen K. Ultrasonography as a tool for diagnosis, guidance of local steroid injection and, together with pressure algometry, monitoring of the treatment of athletes with chronic jumper’s knee and Achilles tendinitis: a randomized, double-blind, placebo-controlled study. Scand J Rheumatol 2004;33(2):94–101 47. Fredberg U, Ostgaard R. Effect of ultrasound-guided, peritendinous injections of adalimumab and anakinra in chronic Achilles tendinopathy: a pilot study. Scand J Med Sci Sports 2009;19(3):338–344 48. Alfredson H, Öhberg L. Sclerosing injections to areas of neovascularisation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc 2005;13(4):338–344 49. Öhberg L, Alfredson H. Sclerosing therapy in chronic Achilles tendon insertional pain—results of a pilot study. Knee Surg Sports Traumatol Arthrosc 2003;11(5):339–343 50. Lind B, Öhberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year followup. Knee Surg Sports Traumatol Arthrosc 2006;14(12): 1327–1332 99 Downloaded by: NYU. Copyrighted material. IMAGING OF ACHILLES TENDINOPATHY AND PARATENDINITIS/WIJESEKERA ET AL SEMINARS IN MUSCULOSKELETAL RADIOLOGY/VOLUME 15, NUMBER 1 51. Willberg L, Sunding K, Öhberg L, Forssblad M, Fahlström M, Alfredson H. Sclerosing injections to treat midportion Achilles tendinosis: a randomised controlled study evaluating two different concentrations of Polidocanol. Knee Surg Sports Traumatol Arthrosc 2008;16(9):859–864 52. Boesen MI, Torp-Pedersen S, Koenig MJ, et al. Ultrasound guided electrocoagulation in patients with chronic noninsertional Achilles tendinopathy: a pilot study. Br J Sports Med 2006;40(9):761–766 53. Chan O, O’Dowd D, Padhiar N, et al. High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil 2008;30(20-22):1697–1708 54. Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 2005;87(1): 187–202 55. Maxwell NJ, Ryan MB, Taunton JE, Gillies JH, Wong AD. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. AJR Am J Roentgenol 2007;189(4): W215–220 56. James SL, Ali K, Pocock C, et al. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med 2007;41(8):518–521; discussion 522 57. Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg Am 2003; 28(2):272–278 58. Testa V, Capasso GM, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc 2002;34(4):573–580 59. McLauchlan GJ, Handoll HH. Interventions for treating acute and chronic Achilles tendinitis. Cochrane Database Syst Rev 2001;2(2):CD000232 60. Brown R, Orchard J, Kinchington M, Hooper A, Nalder G. Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. Br J Sports Med 2006;40(3): 275–279 61. Nelen G, Martens M, Burssens A. Surgical treatment of chronic Achilles tendinitis. Am J Sports Med 1989;17(6): 754–759 62. Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int 1997;18(9):565–569 63. Kvist H, Kvist M. The operative treatment of chronic calcaneal paratenonitis. J Bone Joint Surg Br 1980;62(3):353–357 2011 64. Clancy WG Jr, Neidhart D, Brand RL. Achilles tendonitis in runners: a report of five cases. Am J Sports Med 1976;4(2): 46–57 65. Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc 2003;11(5):334–338 66. Andersson G, Danielson P, Alfredson H, Forsgren S. Nerverelated characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc 2007;15(10):1272–1279 67. Den Hartog BD. Flexor hallucis longus transfer for chronic Achilles tendonosis. Foot Ankle Int 2003;24(3): 233–237 68. Schepsis AA, Leach RE. Surgical management of Achilles tendinitis. Am J Sports Med 1987;15(4):308–315 69. Vora AM, Myerson MS, Oliva F, Maffulli N. Tendinopathy of the main body of the Achilles tendon. Foot Ankle Clin 2005;10(2):293–308 70. Paavola M, Kannus P, Orava S, Pasanen M, Järvinen M. Surgical treatment for chronic Achilles tendinopathy: a prospective seven month follow up study. Br J Sports Med 2002;36(3):178–182 71. Maffulli N, Binfield PM, Moore D, King JB. Surgical decompression of chronic central core lesions of the Achilles tendon. Am J Sports Med 1999;27(6):747–752 72. Paavola M, Orava S, Leppilahti J, Kannus P, Järvinen M. Chronic Achilles tendon overuse injury: complications after surgical treatment. An analysis of 432 consecutive patients. Am J Sports Med 2000;28(1):77–82 73. Steenstra F, van Dijk CN. Achilles tendoscopy. Foot Ankle Clin 2006;11(2):429–438, viii 74. Thermann H, Benetos IS, Panelli C, Gavriilidis I, Feil S. Endoscopic treatment of chronic mid-portion Achilles tendinopathy: novel technique with short-term results. Knee Surg Sports Traumatol Arthrosc 2009;17(10):1264–1269 75. Maquirriain J, Ayerza M, Costa-Paz M, Muscolo DL. Endoscopic surgery in chronic achilles tendinopathies: a preliminary report. Arthroscopy 2002;18(3):298–303 76. Vega J, Cabestany JM, Golanó P, Pérez-Carro L. Endoscopic treatment for chronic Achilles tendinopathy. Foot Ankle Surg 2008;14(4):204–210 Downloaded by: NYU. Copyrighted material. 100