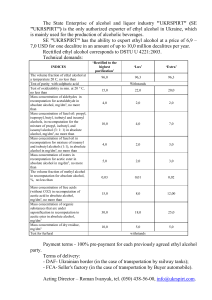
WATER QUALITY CONTROL PART II. Parameters of water
advertisement

WATER QUALITY CONTROL Part II. Parameters of water. WATER QUALITY CONTROL PART II. Parameters of water 39 WATER QUALITY CONTROL Part II. Parameters of water. 2. Natural Waters Natural waters, occurring in the environment, are not chemically pure waters. While circulating in the environment water contacts with atmosphere, rocks and soil. In this way many different compounds pass into the water, either inorganic (mineral) or organic. From physicochemical point of view natural waters are: a. Multicomponent mixtures containing, apart from the solvent, many dissolved substances, or b. Multiphase mixtures containing dispergated solid phase (e.g. suspensions) and liquid phase (e.g. emulsions). In aquatic environment naturally occurring compounds are called admixtures (e.g. Ca2+, Mg2+, Na+, K+, HCO3-, Cl-, SO42- ions, humic substances). The quantity and quality of admixtures depend on the environment of water circulation. The substances of foreign origin are called pollutants [4]. • Inorganic material in natural waters The quantity of inorganic compounds dissolved in natural waters is differentiated. At the two common extremes, rainwater may contain as little as a few milligrams of dissolved material per litre whereas seawater contains approximately 35 g of salt per litre. The presence of even a few milligrams of dissolved matter in a litre of rainwater is derived from a number of sources: sulphate from the oxidation of hydrogen sulphide, sulphur dioxide, and dimethylsulphide, and nitrogenous material from terrestrial vegetation and combustion emissions. After falling onto the land, rainwater moves both through and over the soil and underlying rocks dissolving material as it travels. Such a route may increase the inorganic content of the water. Organic matter is also picked up during this process but can be removed from the water if it subsequently travels through the groundwater system [4]. • Organic material in natural waters Generally all natural waters contain organic matter. Some of these are living organisms which size range from that of bacteria to whales; some is decaying matter, and some has been introduced from external sources such as drainage, factory pollution, and atmospheric fallout. The naturally dissolved organic matter is made up of the transformation products of biogenic material and biological excretion products. 40 WATER QUALITY CONTROL Part II. Parameters of water. Proteins, amino acids, fats, lignines, etc., the structural elements of plants and animals, are being broken down continuously both whilst the organism is living and as it decays. The products of degradation and the compounds which are formed by the reassociation of these fragments, create in water a complex mix of dissolved organic compounds. Organic compounds are secreted and excreted by all living organisms and these in turn are broken down into smaller species by bacteria and chemical processes. This organic matter covers the full range of molecular weight, ranging from totally soluble low molecular weight organics to high molecular weight polymers of colloidal and particulate nature. a. Small organic compounds In the absence of pollution sources, small organic molecules such as amino acids, sugars, fats, and chlorophyll are derived from animal and plant metabolism and the decomposition of larger biologically derived molecules. These in turn are a major source of nutrition for other organisms. In addition to the naturally occurring compounds there are many which are present as a result of man's activities, examples of which include pesticides such as DDT and the polynuclear aromatic hydrocarbons resulting from combustion. The chemical formulas of exemplary small organic compounds are presented below, in Fig.2.1. Fig.2.1. Some of the smaller organic molecules found in natural waters [4]. 41 WATER QUALITY CONTROL Part II. Parameters of water. b. Higher molecular weight compounds Much of the dissolved organic matter is of high molecular weight and falls under the title of humic matter. Humic matter is a complex mixture of polymeric material. Its solubility and metal complexing abilities are largely determined by it containing a large number of phenolic and carboxylic acid groups [4]. 2.1. Natural processes affecting the water composition Any natural water body is a homogenous water solution. Due to circulation in the environment water continuously undergoes physicochemical transformations from solid, liquid to gaseous phase, which greatly affect its composition. While travelling in the environment water contacts different media – soil, rocks and atmosphere, collects and transports dissolved and insoluble, organic and inorganic compounds. Also biological processes and activity of organisms contributes to changes in natural waters composition. When considering composition of natural waters the processes of great importance are weathering, sedimentation, absorption and evaporation (explained in chapter 1 “Introduction to Water Quality Control”, point 1.3.1.) and self purification. 2.1.1. Sedimentation and Dissolution The composition of natural waters is rarely governed purely by homogeneous reactions; there is a continuous cycling of material between the dissolved and solid phases brought about by a combination of chemical, physical, and biological processes. Fig.2.2. The interchange of material between the sediments and water [4]. Some of the solid matter which is suspended in a natural water, or deposited to make up the top layers of the bottom sediments, is material which has been recently deposited from solution. This may arise from a purely chemical process such as precipitation or colloidal 42 WATER QUALITY CONTROL Part II. Parameters of water. aggregation, or may result from the production of solid biomass, such as that which results from the photosynthetic activity of algae. Some of this solid material will be rapidly returned to the dissolved phase by changes in solution chemistry or bacterially assisted decomposition; the remainder will eventually be incorporated into the sediments. Fig.2.2 illustrates the interaction between dissolved and solid phases. Most sediment does not consist of one pure component but are made up of mixtures of clay, silt, sand, minerals, and organic matter. They may be present as a result of local physical, chemical, or biological processes or may have been washed into the water body from external sources. The settlement of solid material out of solution onto the floor of a lake or ocean results in the build up of layers of sediment. If this is not disturbed by movement of the water, distinct layers can result giving a history of the sediment deposition. In other more turbulent regions, such as the mouths of estuaries, the energy of the incoming tide can result in the remobilization of significant depths of sediment during each tidal cycle [4]. 2.1.2. Weathering The intimate contact of rocks and minerals with water is a major factor in their erosion and the dispersal of solid material in soils and the river, lake, and coastal marine bottom and suspended sediments. The increased surface area resulting from the breakdown into smaller particles can enhance the rate of dissolution and change by exposing new surfaces to attack. This attack not only releases material into the water but can radically change the nature of the rocks and minerals. There are two main mechanisms which are responsible for these weathering effects [4]: • Hydrolysis - the hydrogen ions are required for the reaction to occur; hydrogen ions originate from water • Oxidation - for the reaction to occur at least one of the elements in the mineral structure must be in a lower oxidation state which can be readily oxidized. Whilst many elements fulfil this criterion the most commonly encountered examples contain iron (II), manganese (II) and sulphide. Weathering will produce an increase in inorganic salt content. The water composition may be also affected by interaction with material on the bed of water reservoir. 2.1.3. Colloids and Their Aggregation Lying between the dissolved state, and particles which precipitate out of solution, there is a class of very small particles suspended in water, which range in diameter between 1 nm and 43 WATER QUALITY CONTROL Part II. Parameters of water. 1µm. These are colloids. They play a significant role in the aquatic chemistry of both inorganic and organic compounds. Because they are suspended in the water they are transported along with moving water bodies. Once destabilized however they rapidly come together to produce larger particles that can precipitate and drop to the bottom sediments, carrying with them other adsorbed and co-precipitated material. The particles are not visible to the naked eye and a colloidal sol (the colloidal equivalent of a solution) appears completely homogeneous. When allowed to stand, colloids do not settle out like heavier particles. Colloidal particles are charged and therefore move in an electric field. This primary charge may be positive or negative and may change with the solution pH. In aqueous solution however, there can be no overall charge and the primary charge must therefore be counterbalanced by the collection of opposite charges around the particles - a double layer therefore exists at the interface between the solid and the water [4]. 2.1.4. Adsorption and Absorption Adsorption is the preferential partitioning of substances from the gaseous or liquid phase onto the surface of a solid substrate [23]. The binding to the surface is usually weak and reversible [22]. The process of adsorption involves separation of a substance from one phase accompanied by its accumulation or concentration at the surface of another. The adsorbing phase is the adsorbent, and the material concentrated or adsorbed at the surface of that phase is the adsorbate. Adsorption is thus different from absorption, a process in which material transferred from one phase to another (e.g. liquid) interpenetrates the second phase to form a "solution". Absorption means the penetration of a substance into the body of another. In natural waters living organisms absorb oxygen, carbon dioxide and nutrients necessary for growth [23]. 2.1.5. Natural Purification Precipitation that falls onto the surface passes through the layers of soil and infiltrate into the ground. Due to physical, chemical and biological processes (Table 2.1.) water passing through the ground undergoes purification. The speed of filtration is very low, that is why the processes of biochemical and sorption self-purification (spontaneous decrease in degree of pollution) are complete and ground waters do not reveal physico-chemical and bacteriological pollution [2]. 44 WATER QUALITY CONTROL Part II. Parameters of water. Table 2.1. Self-purification processes concerning ground waters. Physical processes Chemical processes Biological processes Dilution Coagulation Precipitation Sorption (adsorption) Ion-exchange Filtration Degradation Oxidation Reduction Hydrolysis Biodegradation Decaying In case of surface waters they, in time, undergo self-purification as a result of biochemical processes, due to microbiological activity of organisms in presence of oxygen and sedimentation. Microorganisms activity leads to decomposition of organic compounds into simple inorganic ones (carbon dioxide, sulphates, nitrates and water), dilution or removal of pollutants from water by living organisms. Self-purification processes occur with various speeds in different environment conditions. The intensity depends on degree of aeration of water reservoir, microorganisms’ content and type of pollutants [2]. 2.2. Classes of Natural Waters The quality of surface waters is not equal and even, and has to be established. Standards of permissible pollution of surface waters and conditions of sewage disposal are regulated according to The Cabinet Decree form 9 June 1970. In Poland surface waters (inland flowing waters) are divided into 3 classes of cleanness, on the basis of established norms [13]: a. I class - the cleanest waters; water capable to use as a drinking water directly; waters suitable for the use in branches of industry requiring high quality water (food and pharmaceutical industry); water which fulfil requirements for ponds with salmon b. II class - waters suitable for fish other than salmon, raising of farm animals, cultivation and recreation c. III class - waters suitable for the use in industry other than industry which require I class water; for watering of agricultural and garden areas. Waters exceeding norms for the III class water are called pose-class waters. The standards of acceptable surface water pollutants are presented in Table 2.2. 45 WATER QUALITY CONTROL Part II. Parameters of water. Table 2.2. Standards of acceptable inland surface waters pollution [13]. No. Indicator or kind of pollution 1. Dissolved oxygen Biological Oxygen Demand (BOD5) Chemical Oxygen Demand (COD), (permanganate Chemical Oxygen Demand (COD), dichromate 2. 3. 4. Concentration Unit mg O2/dm3 I 6 and above Clarity class II 5 and above III 4 and above mg O2/dm3 4 and below 8 and below 12 and below mg O2/dm3 10 and below 20 and below 30 and below mg O2/dm3 40 and below 60 and below 100 and below Oligo to Betamezzo to betamezzo alfamezzo 250 and below 300 and below 150 and below 200 and below 350 and below 550 and below 1000 and 500 and below below 400 and below 250 and below 700 and below 1200 and below 20 and below 50 and below 5. Saprobythes mg O2/dm3 6. 7. 8. Chlorides Sulphates Total hardness mg Cl/dm3 mg SO4/dm3 G CaCO3/dm3 9. Dissolved substances mg/dm3 mg/dm3 11. 12. 13. 14. 15. 16. 17. Turbidity (total suspensions, except for sudden water rise) Ammonia salts Nitrates pH Organic nitrate Total iron Manganese Phosphates 18. Rhodanic acids mg CNS/dm3 19. Cyanides (except for complexes) mg CN/dm3 20. Cyanide complexes mg MeCNx/dm3 mg/dm3 21. Volatile phenols 10. 22. 23. Surfactants Temperature1 24. Odour 25. 26. 27. 28. 29. 30. 31. 2 Colour Faecal Coliforms Factor Pathogenic bacteria Oils Ether extract Lead Mercury 3 mg NH4/dm mg NO3/dm3 mg Norg/dm3 mg Fe/dm3 mg Mn/dm3 mg PO4/dm3 1 and below 3 and below 6 and below 1.5 and below 7 and below 15 and below 6.5-8.5 6.5-9.0 6.0-9.0 1 and below 2 and below 10 and below 1.0 and below 1.5 and below 2.0 and below 0.1 and below 0.3 and below 0.8 and below 0.2 and below 0.5 and below 1.0 and below 0.02 and 0.1 and below 2.0 and below below 0.01 and 0.02 and below 0.05 and below below 1.0 and below 0.005 and below 1 and below 22 and below 3 mg/dm °C 3R and below 3 mg Pt/dm 30 and below Alfamezzo 2.0 and below 3.0 and below 002 and below 0.05 and below 2 and below 26 and below natural 3 and below 26 and below Slight at the very most natural 0.1 and above 0.01 and above Undetectable Invisible on water surface 5 and below 15 and below 40 and below 0.1 and below 0.1 and below 0.1 and below 0.005 and 0.005 and 0.01 and below 1 and above mg/dm3 mg Pb/dm3 mg Hg/dm3 1 If natural water temperature is equal to or higher than standard value for particular clarity class of water, the temperature increase is possible of 2°C 2 In special cases it is possible to change (increase) the value of Pt indicator, for I and II class not more than of 15 mg/dm3 Pt, and for III class not more than of 30 mg/dm3 Pt. 46 WATER QUALITY CONTROL Part II. Parameters of water. No. Indicator or kind of pollution Concentration Unit 32. Copper mg Cu/dm3 33. Zink mg Zn/dm3 34. Cadmium mg Cd/dm3 35. 36. 37. Chromium (III) Total heavy metals Nickel mg Cr/dm3 mg/dm3 mg Ni/dm3 38. Chromium (VI) mg Cr/dm3 39. Silver mg Ag/dm3 40. 41. Vanadium Boron mg V/dm3 mg B/dm3 42. Arsenic mg As/dm3 43. 44. 45. 46. 47. 48. 49. Chloride Fluoride Sulphites Ammonia Acrylnitrile Caprolactam Radioactive substances mg Cl/dm3 mg F/ dm3 mg S/dm3 mg NH3/dm3 mg/dm3 mg/dm3 50. Biological test with fish 24 hours Clarity class I II III below below 0.01 and 0.1 and below 0.2 and below below 0.01 and 0.1 and below 0.2 and below below 0.005 and 0.03 and below 0.1 and below below 0.5 and below 0.5 and below 0.5 and below 1.0 and below 1.0 and below 1.0 and below 1.0 and below 1.0 and below 1.0 and below 0.05 and 0.1 and below 0.1 and below below 0.01 and 0.01 and below 0.01 and below below 1.0 and below 1.0 and below 1.0 and below 1.0 and below 1.0 and below 1.0 and below 0.05 and 0.05 and below 0.2 and below below Undetectable 1.2 and below 1.2 and below 2.0 and below undetectable 0.1 and below 0.1 and below 0.1 and below 0.1 and below 2.0 and below 2.0 and below 2.0 and below 1.0 and below 1.0 and below 1.0 and below In quantity determined by different regulations Positive, water should not cause dead of fish during 24 hours The acceptance of particular class of water is made by water administration organ. If water is for different use the class of water is that of higher requirements. Drinking water regulations are specified by Decree of Minister of Health and Social Welfare from 4 May 1990 and are stricter in case of some compounds present in water. They are listed in chapter 4 “Water for Different Purposes”, point 4.1. To estimate the class of water it is necessary to make an analysis of physicochemical and biological water parameters. 2.3. Parameters of Natural Waters Determination of water parameters is used for description of the quality of water. Nowadays European countries use 64 parameters to determine water parameters and its quality. Polish rules require only 51 parameters. The strictest rules considering water parameters are established by EPA (Environmental Protection Agency 1995). It is equal to 120 parameters. 47 WATER QUALITY CONTROL Part II. Parameters of water. Tests for the physical/chemical parameters monitor the characteristics that affect the appearance, taste and odour of water but generally do not cause a health risk, while microbiological tests are for waterborne organisms that could potentially cause disease. 2.3.1. Organoleptic Parameters a. Colour Colour is an optical parameter consisting in absorbing of a part of spectrum of visible radiation by substances dissolved in water, colloidal substances, and suspended particles present in water or sewage. Colour in water may appear as the result of different sources activity. These are: • Natural Sources: type of vegetation, decay of plant matter, humic substances, algae growth, plankton, minerals (iron, manganese and copper). • Anthropogenic sources: sewage from paper mills, textile mills, food processing There are two types of water colour considered: "apparent colour” and "true colour". True colour is distinguished from apparent colour by filtering the sample. True colour is mostly found in surface water, although ground water may contain some colour if the aquifer flows through a layer of buried vegetation, such as from a long buried slough of a river. Apparent colour is caused by coloured suspensions and dissolved matter. The unit of colour is the colour developed in 1 dm3 of distilled water by 1mg of dissolved platinum (potassium hexachloroplatinum (IV) (K2PtCl6)) with addition of 0.5 mg of cobalt (cobalt chlorate (II) CoCl2 · 6H2O). Colour measurements are carried out by the use of optical principle, light absorbency and place detector in direction of incoming light source. Colour can be removed by activated carbon filters, sometimes marketed as taste and odour filters. Another treatment method is coagulation and sedimentation using alum or other chemicals. There is no direct link between colour and health effects and the colour of water is usually only an aesthetic problem, both in drinking water and wastewater. However, water colour may be also an indicator of toxicity and may stain textiles and fixtures. It may be result of the presence of coloured organic substances, metals (Fe, Mg and Cu) or industrial wastes. Colour is vital as most water users, be it domestic or industrial, usually prefer colourless water. 48 WATER QUALITY CONTROL Part II. Parameters of water. According to polish regulations colour in drinking water must not exceed 20 mg(Pt)/dm3. In case of ground water it should not exceed 25 mg(Pt)/dm3, and in case of surface waters it should be true colour. Natural waters are yellowish-green in colour. Waters flowing out of marshy and forest areas, rich in humic substances, are yellowish-brown. Such colour originates from natural humic substances and decomposition of plant material taking place in soil, swamps or peat [10]. b. Turbidity Turbidity is an expression of the optical properties that cause light to be scattered and absorbed rather than transmitted in a straight line through the water. Turbidity in water is caused by the presence of dissolved inorganic and organic particles, like: • Soluble coloured compounds (iron, manganese and aluminium), • Humic acids, • Plankton and microscopic organisms (also bacteria and other pathogens), • Clay and silt, • Suspensions from sewage disposal. Turbidity depends on a number of factors, such as the quantity, size, shape, and refractive index of the particles and the wavelength of the incident light [1]. Particles that cause turbidity in water vary in size between 1 nm and 1 mm [6]. They can be divided into three classes: • Clay particles, which have an upper particle size limit of about 0.002 mm diameter; produced by the erosion of the land surface constitute the major part of the suspended material in most natural waters [7], • Organic particles, produced by the decomposition of plant and animal debris; Organic particulates may harbour microorganisms. Thus, turbid conditions may increase the possibility for waterborne disease. Nonetheless, inorganic constituents have no notable health effects, • Fibrous particles, e.g. those of minerals such as asbestos. All natural waters are turbid, surface waters generally more than subsurface waters. Groundwater is usually totally clear due to filtration property of soil. However, after excavation it very often clouds up, especially in contact with air. This is the result of the change in chemical composition of water: a part of dissolved carbon dioxide (CO2) passes to atmosphere causing the calcium-carbon equilibrium to be upset. This leads to gradual 49 WATER QUALITY CONTROL Part II. Parameters of water. precipitation of calcium, magnesium and iron carbonates and hydroxides, in the following reactions: Ca(HCO3) 2 ∆ CaCO3 (↓) + CO2 (↑) +H2O Fe(HCO3) 2 +2H2O ∆ Fe(OH) 2 (↓) + 2H2CO3 2Fe(OH) 2 + ½ O2 + H2O = 2Fe(OH) 3 Usually after a few hours there is a total sedimentation of precipitates and water becomes clear again [10]. The series of turbidity-induced changes that can occur in a water body may change the composition of an aquatic community. First, turbidity due to a large volume of suspended sediment will reduce light penetration, thereby suppressing photosynthetic activity of phytoplankton, algae, and macrophytes, especially those from the surface. If turbidity is largely due to algae, light will not penetrate very far into the water, and primary production will be limited to the uppermost layers of water. Overall, excess turbidity leads to fewer photosynthetic organisms available to serve as food sources for many invertebrates. As a result, overall invertebrate numbers may also decline, which may then lead to a fish population decline. If turbidity is largely due to organic particles, dissolved oxygen depletion may occur in the water body. The excess nutrients available will encourage microbial breakdown, a process that requires dissolved oxygen. In addition, excessive nutrient content may result in algal growth. Although photosynthetic by day, algae respire at night, using valuable dissolved oxygen. Fish kills often takes place because of extensive oxygen depletion. High turbidity can also reduce the growth of clams and oysters; it can slow or stop egg development. The comparative unit of turbidity is turbidity developed by 1 mg of silica (SiO2) added to 1 dm3 of distilled water. Acceptable turbidity of drinking water consistent with polish regulations must not exceed 5 mg/dm3. For surface waters the requirements are: I class - 20 mg/dm3 II class - 30 mg/dm3 II class - 50 mg/dm3 Because of microbiological effects it is recommended that turbidity be kept as low as possible. 50 WATER QUALITY CONTROL Part II. Parameters of water. • Turbidity in drinking water Turbidity can have a significant effect on the microbiological quality of drinking-water. Its presence can interfere with the detection of bacteria and viruses in drinking water [5]; moreover, turbid water has been shown to stimulate bacterial growth [6] since nutrients are adsorbed onto particulate surfaces, thereby enabling the attached bacteria to grow more rapidly than those in free suspension. The adsorptive capacity of some suspended particulates can lead to the presence of undesirable inorganic and organic compounds in drinking-water. Most important in this respect is the organic or humic component of turbidity. In addition, the strength of the bonds in some metal–humate complexes in the turbidity fraction may complicate the measurement of trace metals in natural waters, resulting in an underestimation of the metal concentrations. Turbid water is not suitable for consumption. The consumption of highly turbid water may constitute a health risk, because excessive turbidity can protect pathogenic microorganisms from the effects of disinfectants, stimulate the growth of bacteria in distribution systems, and increase the chlorine demand. In addition, the adsorptive capacity of some particulates may lead to the presence of harmful inorganic and organic compounds in drinking-water. c. Suspended Solids Solids present in water can be divided into three types according to size: • Suspended > 1 mm (larger than bacteria) • Colloidal between 1 mm and 0.001 mm • Dissolved < 0.001 mm The suspended solids include sand, silt, rust, plant fibres and algae and are an indicator of possible bacterial or hazardous contamination. Total Suspended Solids is the mass of solids that can be separated from the water by filtration. Total Suspended Solids is an indication of the amount of erosion that took place nearby or upstream. It can also be caused by plankton growth, or wastewater. d. Dry residues Dry residue is a residue left after evaporation of water, drying in 105°C and recalculation into 1 dm3 of water or sewage. It makes up for the mass of dissolved and insoluble inorganic and organic substances present in water. High content of dry residue contribute to the bitter water taste and have negative impact on the organisms as regards to physiology. 51 WATER QUALITY CONTROL Part II. Parameters of water. It is advisable that drinking water content of dry residue would not exceed 500 mg/dm3 [2]. e. Taste and odour Chemically clean water is odourless, while odour of natural waters depends on the type and quantity of dissolved substances [10]. Taste and odour are usually inter-related. Compounds in water that are perceived as giving it a taste are generally inorganic substances present at concentrations much higher than those of organic pollutants. Inorganic chemicals that can affect taste but not cause any odour are salt (NaCl), minerals and metals. The salt concentration in water should be approximately the same as in saliva for the water to taste neutral. Of the ions that may be present in water, iron can be tasted in distilled water at concentration of about 0.05 mg/dm3, copper at about 2.5 mg/dm3, manganese at about 3.5 mg/dm3, and zinc at about 5 mg/dm3. Iron, in particular, is suspected of affecting the taste of water in practice [14]. A few inorganic chemicals can cause both taste and odour problems. These are ammonia, chlorine and hydrogen sulphide. Organic chemicals usually affect both taste and odour: The compounds concerned include humic substances, hydrophilic Table 2.3. The threshold odour for some chemical contaminants. acids, carboxylic acids, peptides and Compound Threshold [mg/dm3] amino acids, carbohydrates, and Chlordane 0.0003 hydrocarbons [14], biological decay 1,4-dichlorobenzene 0.0003 products, petroleum products and Trichloroethylene 0.5 Phenol 1 - 15.9 4-chlorophenol 0.0005 - 1 2,4-dichlorophenol 0.002 - 0.32 Hydrogen cyanide 0.001 pesticides. These compounds are detectable at extremely concentrations (Table 2.3). low The organisms most often linked to taste and odour problems are actinomycetes and various types of algae, but other aquatic organisms, such as protozoa and fungi, have been implicated from time to time. Earthy-musty tastes and odours are produced by certain cyanobacteria (blue-green algae), actinomycetes, and a few fungi. Growing algae produce numerous volatile and non-volatile organic substances, including aliphatic alcohols, aldehydes, ketones, esters, thioesters, and sulphides. 52 WATER QUALITY CONTROL Part II. Parameters of water. Occasionally, taste and odour problems in water are caused by other bacteria, fungi, zooplankton, and nemathelminthes. Ferrobacteria in water-distribution systems may produce tastes and odours, and some species of Pseudomonas can cause a swampy odour, whereas others can convert sulphur-containing amino acids into hydrogen sulphide, methylthiol, and dimethylpolysulfide [14]. Ground waters are usually odourless, however, sometimes they posses a characteristic odour of hydrogen sulphide (H2S) originating from decomposition of sulphide minerals in presence of dissolved carbon dioxide [10]: FeS2 + 2CO2 + 2H2O → Fe(HCO3) 2 + H2S + S MnS + 2CO2 + 2H2O → Mn(HCO3) 2 + H2S • Taste and odour in drinking water Taste and odour in drinking water can be caused by microorganisms or may be of human origin. Problems can also be caused by some water-treatment processes or by substances leached from water pipes or storage facility linings. Odour in potable water may be indicative of some form of pollution of water, malfunction during water treatment process or distribution of water. It should not be accepted without knowledge of the exact cause. Water treatment often includes storage, slow sand filtration or activated carbon filtration. Microorganisms can grow in the equipment used for these purposes and can then cause tastes and odours. The biological degradation of organic compounds in raw water can also lead to the production of substances such as phenols, aldehydes, and alkylbenzenes that cause taste and odour problems. In addition, the chemicals used in water treatment as coagulants, oxidants, or disinfectants can interact with organic compounds in water and occasionally produce tastes and odours [14]. Ozone is one of the most efficient agents in removing tastes and odours, but its use can lead to the formation of intermediate reaction products. In particular the formation of aliphatic aldehydes, which has been frequently reported in the literature, leads to the development of fruity, fragrant, and orange-like odours. The free halogens used as water disinfectants can produce undesirable tastes and odours in the water. Taste and odour problems that develop in water-treatment plants are frequently an indirect consequence of chlorination [14]. 53 WATER QUALITY CONTROL Part II. Parameters of water. 2.3.2. Physicochemical Parameters a. Temperature Distribution of temperature is different for surface waters and groundwater. Temperature of surface waters depends mainly on: • Water origin, • Climatic zone, • Season, • Altitude, • Degree of riparian coverage, • Inflow of industrial and municipal sewage (power plants, industrial cooling) [10]. Water temperature can fluctuate diurnally and seasonally, however, daily variation of air temperature can hardly affect water temperature. Flowing waters usually have unique temperature depending on flow velocity. In case of steady reservoirs variations in temperature can occur due to thermal stratification and restricted mixing of layers (stratification tends to persist until cooler fall weather). Water layers with dissimilar temperature differ in density and oxygen content. Temperature of groundwater depends mainly on the depth of water occurrence. In Poland temperature of groundwater increases ca.1°C every 33 m going down, due to the increase of soil temperature. Temperature can exert great control over aquatic communities, especially influence on biological activity and growth. If the overall water body temperature of a system is altered, an aquatic community shift can be expected. An increase of 10°C in water temperature almost doubles the speed of chemical and biological reactions occurring in water. Temperature increase leads to: • Decrease the amount of dissolved oxygen (DO) • Increase biochemical oxygen demand (BOD) • Acceleration of nitrification and oxidation of ammonia to nitrates (III) and (V) which eventually lead to oxygen deficit in water. Higher temperature also increases toxicity of many substances (pesticides, heavy metals) and susceptibility of organisms to toxicants. Organisms (including fish) are also sensitive to 54 WATER QUALITY CONTROL Part II. Parameters of water. temperature, as must migrate through changing temperature zones. Sudden temperature changes affects fish more than extremes. Acceptable temperature of water in Poland is equal to: • surface water I class - 22°C II and III class - 26°C Temperature of drinking water is not regulated. It does not influence hygienic value of drinking water; however it is important for taste. Constant temperature 7 - 12°C is recommended. b. pH The pH is a measure of the acidity of a solution (H+ ions) and ranges in scale from 0 to 14 (from very acidic to very alkaline). Water dissociation reaction (see chapter 1, point 1.1.1) indicates that chemically pure water is neutral. The pH values of natural waters (Table 2.4) are influenced by different the presence of admixtures (i.e. carbons, hydrocarbons, carbon dioxide, sulphur dioxide etc.) which alter neutral pH of water. The important factors are geological characteristics of bottom of a water body, which can contain acidic or basic compounds as well as vegetation, land use practices, sewage inflow and atmospheric precipitation. Table 2.4. pH of natural waters Water type Surface water Groundwater Acid rain Lakes damaged by acid rain pH value 6.5 - 8 5.5 - 7.5 as low as 3 4 or less Water pH is crucial for living organisms, biochemical processes and industrial water use. The pH is indicator of the existence of biological life as most of them thrive in a quite narrow and critical pH range. In too acidic or too basic waters biological life extinct. Low water pH accelerates heavy meats being washed away from sediments. Acidic waters are highly corrosive. According to polish standards permissible values of water pH are the following: • drinking water • surface water I class - 6.5 – 8.5 - 6.5 – 8.5 55 WATER QUALITY CONTROL Part II. Parameters of water. II class - 6.5 – 9.0 III class - 6.0 – 9.0 c. Alkalinity Alkalinity refers to the capability of water to neutralize acids. This is really an expression of buffering capacity. A buffer is a solution to which an acid can be added without changing the concentration of available H+ ions (without changing the pH) appreciably. It essentially absorbs the excess H+ ions and protects the water body from fluctuations in pH [20]. Generally, the basic species responsible for alkalinity in water are bicarbonate ion, carbonate ion and hydroxide ion [3]: HCO3- + H+ → CO2 + H2O CO32- + H+ → HCO3OH- + H+ → H2O The presence of calcium carbonate or other compounds such as magnesium carbonate contribute to the buffering system [20]. Minor contributors to alkalinity are ammonia and the conjugate bases of phosphoric, silicic, boric, and organic acids [3]. Alkalinity is often related to hardness because the main source of alkalinity is usually from carbonate rocks (limestone) which are mostly CaCO3. Since hard water contains metal carbonates (mostly CaCO3) it is high in alkalinity. Conversely, unless carbonate is associated with sodium or potassium which do not contribute to hardness, soft water usually has low alkalinity and little buffering capacity. So, generally, soft water is much more susceptible to fluctuations in pH from acid rains or acid contamination [20]. It is important to distinguish between high basicity, manifested by an elevated pH, and high alkalinity, the capacity to accept H+. Whereas pH is an intensity factor, alkalinity is a capacity factor [3]. Alkalinity (as well as pH) can be determined using inexpensive test strips. However, more sophisticated electrometric measurement is performed by the computer aided titrimeter (CAT) and the pH electrode. Alkalinity is important for fish and aquatic life because it protects or buffers against rapid pH changes. Living organisms, especially aquatic life, function best in a pH range of 6.0 to 9.0. For protection of aquatic life the buffering capacity should be at least 20 mg/dm3 [20]. 56 WATER QUALITY CONTROL Part II. Parameters of water. d. Acidity Acidity of natural water systems is the capacity of water to neutralize hydroxide ions OH-. Acidity is generally due to the presence of weak acids such as H2PO4-, CO2, H2S, proteins, fatty acids, and acidic metal ions, particularly Fe3+. Factors causing acidity of natural waters can originate from atmosphere (e.g. CO2), soil (CO2 and humic acids), coagulants added to water during treatment and from industrial sewage inflow. The term free mineral acid is applied to strong acids such as H2SO4 and HCl in water. Whereas total acidity is determined by titration with base to the phenolphthalein endpoint (pH 8.2, where both strong and weak acids are neutralized), free mineral acid is determined by titration with base to the methyl orange endpoint (pH 4.3, where only strong acids are neutralized). The acidic character of some hydrated metal ions may contribute to acidity as shown by the following example: A1(H2O)63+ + H2O ∆ A1(H2O)5OH2+ + H3O+ Some industrial wastes, for example, pickling liquor used to remove corrosion from steel, contain acidic metal ions and often some excess of strong acid [3]. e. Conductivity Conductivity is a measure of the capacity of an aqueous solution to carry an electrical current. Conductivity depends on the presence of ions (cations and anions) in water, their total concentration, mobility and valence, and on temperature of water. In natural waters ions usually origin from inorganic compounds present in water. Organic compounds dissociate to minimal degree or do not dissociate at all. Conductivity is a good measure of the total amount of salts in water (e.g., calcium, magnesium, sodium, potassium, carbonate, bicarbonate, sulphate, chloride, nitrate, and others). It is commonly used to determine salinity [6]. 57 WATER QUALITY CONTROL Part II. Parameters of water. High salinity may interfere with the growth of aquatic vegetation. Salt may decrease the osmotic pressure, causing water to flow out of the plant to achieve equilibrium. Less water can be absorbed by the plant, causing stunted growth and reduced yields. High salt concentrations may cause leaf tip and marginal leaf burn, bleaching, or defoliation. According to polish standards permissible conductivity of drinking water is 1500 mS/cm, and acceptable amounts of substances dissolved in water are: • drinking water • surface water - should not exceed 800 mg/dm3 I class - 500 mg/dm3 II class - 1000 mg/dm3 III class - 1200 mg/dm3 f. Hardness The hardness of water is the concentration of ions that will react with a sodium soap to precipitate an insoluble residue. Water hardness is the result of dissolved minerals presence, usually total concentration of cations of calcium Ca2+, magnesium Mg2+, iron Fe3+ and manganese Mn2+. The reaction for calcium is: 2C17H35COO-Na+ + Ca2+ → Ca(C17H35CO2)2 (↓)+ 2Na+ The following types of water hardness are under consideration [2]: • Total (temporary) hardness - total amount of calcium and magnesium ions (or other metals) responsible for water hardness • Carbonate hardness - amount of calcium and magnesium hydrocarbons; disappears while boiling - precipitation of insoluble sediment Ca(HCO3)2 ∆ CaCO3 (↓) + H2O + CO2(↑) Mg(HCO3)2 ∆ MgCO3(↓) + H2O + CO2(↑) MgCO3 + H2O ∆ Mg(OH)2(↓) + CO2(↑) • Non-carbonate hardness -difference between total hardness and carbonate hardness; determine the amount of chlorides, sulphates, nitrates and other soluble salts. Mainly calcium and magnesium salts. 58 WATER QUALITY CONTROL Part II. Parameters of water. Water hardness is given in milimols of calcium and magnesium ions in 1 dm3 of water, 1mmol = 40.08 mg Ca2+ (or 24.32 mg Mg2+) in 1 dm3 of water. It can also be expresses in so called hardness degrees - German, French, and others. 1mmol = 5.61°n (°DH) or 10 °F, 1 German degree (1°n) = 10 mg CaO (or 7.19 mg MgO) in 1 dm3 of water, 1 French degree (1°F) = 10 mg CaCO3 in 1 dm3 of water. According to hardness level water is divided into different categories, listed in Table 2.5. Table 2.5. Typical Hardness Values Hardness Values (mmol/dm3) 0 - 0.89 0.89 – 1.87 1.78 – 2.68 2.68 – 3.57 3.57 – 5.35 >5.35 Water type Very soft Soft Moderately hard Significantly hard Hard Very Hard The effect of hardness mainly consists in causing soap scum and water spots, scaling in swamp coolers, cooling towers, boilers and pipes. Hard waters are satisfactory for human consumption as soft waters. [11] Acceptable water hardness is the following: should not exceed 5 mmol/dm3 (500 mg CaCO3/dm3) • drinking water - • surface water I class - 3.50 mmol/dm3 (350 mg CaCO3/dm3) II class - 5.50 mmol/dm3 (550 mg CaCO3/dm3) III class - 7.00 mmol/dm3 (700 mg CaCO3/dm3) g. Dissolved Oxygen (DO) Dissolved oxygen (DO) refers to the volume of oxygen present in water and it is a basic indicator of ecosystem health. Oxygen enters the water as rooted aquatic plants and algae undergo photosynthesis, and as oxygen is transferred across the air-water interface. The amount of oxygen that can be held by water depends on the water temperature, salinity, and pressure: • Gas solubility increases with decreasing temperature (colder water holds more oxygen) • Gas solubility increases with decreasing salinity (freshwater holds more oxygen than does saltwater) 59 WATER QUALITY CONTROL Part II. Parameters of water. • Gas solubility decreases as pressure decreases, thus, the amount of oxygen absorbed in water decreases as altitude increases because of the decrease in relative pressure. • The partial pressure and the degree of saturation of oxygen will change with altitude. Once absorbed, oxygen is either incorporated throughout the water body via internal currents or is lost from the system. Oxygen losses readily occur when water temperatures rise, when plants and animals respire, and when aerobic microorganisms decompose organic matter. Oxygen levels are also affected by the diurnal cycle. Plants, such as rooted aquatic plants and algae produce excess oxygen during the daylight hours when they are photosynthesizing. During the dark hours they must use oxygen for life processes. Flowing water is more likely to have high dissolved oxygen levels compared to stagnant water because the water movement at the air-water interface increases the surface area available to absorb the oxygen. In flowing water, oxygen-rich water at the surface is constantly being replaced by water containing less oxygen as a result of turbulence. Because stagnant water undergoes less internal mixing, the upper layer of oxygen-rich water tends to stay at the surface. Maximum amount of oxygen in clean water is about 9 mg/dm3. Prolonged exposure to low dissolved oxygen levels (less than 5 to 6 mg/dm3 oxygen) may not directly kill an organism, but will increase its susceptibility to other environmental stresses. Exposure to less than 30% saturation (less than 2 mg/dm3 oxygen) for one to four days may kill most of the aquatic life in a system [24]. Rules include minimum concentrations of dissolved oxygen which must be met in surface waters. Polish regulations define that surface waters must meet a minimum dissolved oxygen standard of: I class - 6 mg/dm3 II class - 5 mg/dm3 III class - 4 mg/dm3 Oxygen content in drinking water is not regulated [2]. h. Biochemical Oxygen Demand (BOD) Biochemical Oxygen Demand, or BOD, is a measure of the quantity of oxygen consumed by microorganisms during decomposition of organic matter. BOD is the most commonly used parameter for determining the oxygen demand on the receiving water of a municipal or industrial discharge. BOD can also be used for evaluation the efficiency of treatment processes, and it is an indirect measure of biodegradable organic compounds in water. High 60 WATER QUALITY CONTROL Part II. Parameters of water. BOD is an indication of poor water quality. The lower the BOD the less organic matter is present in water. A high BOD is often accompanied by a low DO level [25]. BOD is typically divided into two parts: • Carbonaceous Biochemical Oxygen Demand - is the result of the breakdown of organic molecules such a cellulose and sugars into carbon dioxide and water (1st stage of oxidation) HCOH + O2 bacteria → CO2 + H 2 O • Nitrogenous Oxygen Demand - is the result of the breakdown of proteins (2nd stage of oxidation). NH 3 bacteria ,oxygen → NO2− → NO3− It is estimated that it takes 20 days for biodegradation process to be completed [10]. BOD is a parameter commonly measured by determining the quantity of oxygen utilized by suitable aquatic microorganisms during a five-day period and is then called BOD5. Though the choice of a five-day period is somewhat arbitrary, a five-day BOD5 test remains a respectable measure of the short-term oxygen demand exerted by a pollutant [3]. Determination of BOD5 consists in estimation of dissolved oxygen concentration before and after five-day incubation. BOD5 is usually as much as 68 – 82% of total BOD. Water standards for dissolved oxygen include minimum concentrations of dissolved oxygen that must meet the following [2]: I class - 4 mg/dm3 II class - 8 mg/dm3 III class - 12 mg/dm3 i. Chemical Oxygen Demand, COD The Chemical Oxygen Demand (COD) is the amount of oxygen, in mg/dm3, required for degradation of the organic compounds of waste water to occur. The bigger the COD value of waste water, the more oxygen the discharges demand from water bodies [26]. The COD test allows measurement of a waste in terms of the total quantity of oxygen required for oxidation to carbon dioxide and water. It is based upon the fact that all organic 61 WATER QUALITY CONTROL Part II. Parameters of water. compounds, with a few exceptions, can be oxidized by the action of strong oxidizing agents under acid conditions. During the determination of COD, organic matter is converted to carbon dioxide and water, regardless of the biological assimilability of the substances. One of the chief limitations of the COD test is its inability to differentiate between biologically oxidizable and biologically inert organic matter. In addition it does not provide any evidence of the rate at which the biologically active material would be stabilized under conditions that exist in nature. The major advantage of the COD test is the short time required for evaluation. The determination can be made in about 3h rather than 5 days required for the measurement of BOD. For this reason it is used as a substitute for the BOD test in many instances. COD data can often be interpreted in terms of BOD values after sufficient experience has been accumulated to establish reliable correlation factors [12]. However, the values of COD are higher than corresponding values of BOD5, because while making BOD5 test the biodegradation process is not completed [10]. Oxidizing agent that has been found to be the most practical for determination of chemical oxygen demand is potassium dichromate (K2Cr2O7). It is capable of oxidizing a wide variety of organic substances almost completely to carbon dioxide and water. In order for potassium dichromate to oxidize organic matter completely the solution must be strongly acidic and at elevated temperature. Certain organic compounds, particularly low-molecular-weight fatty acids, are not oxidized by dichromate unless a catalyst is present. It has been found that silver ion acts effectively in this capacity. Aromatic hydrocarbons and pyridine are not oxidized under any circumstances [12]. Another oxidizing agent that can be used in COD test, with smaller oxidizing properties, is potassium permanganate (KMnO4) [10]. Presence of chloride ions Cl- greatly affects the results of COD test, as they react with both potassium dichromate and potassium permanganate. The influence is eliminated by addition of mercury sulphate leading to formation of dissociated compounds like (HgCl2)n or [HgCl4] which do not take part in reaction [10]. The rules determining permissible values of COD in waters are the following [2]: • drinking water • surface water3 3 - should not exceed 3 mg O2 /dm3 I class - 10 mg/dm3 II class - 20 mg/dm3 III class - 30 mg/dm3 According to potassium permanganate (KMnO4) determination method 62 WATER QUALITY CONTROL Part II. Parameters of water. j. Carbon dioxide (CO2) Carbon dioxide is present in atmosphere and all kinds of natural waters. The sources of CO2 in natural waters are atmospheric air, degradation of organic compounds, weathering and erosion of rocks and metabolic processes of organisms [10]. Carbon dioxide concentration in water depends on temperature, content of organic compounds and intensity of biochemical processes taking place in water environment. Decrease in CO2 content results in increase of pH and alkalinity, which eventually leads to decrease of solubility of some compounds (e.g. CaCO3, Mg(OH)2). The concentration of carbon dioxide in surface and drinking water is not regulated. It does not influence hygienic value of drinking water; however influences its taste. k. Chlorine Chlorine in elemental form (Cl2) does not exist in natural waters. It can only be delivered with sewage which undergone chlorination with chlorine or chlorinated compounds. Chlorine added to water reacts in the following way [2]: Cl2 + H2O ∆ H+ + Cl- + HOCl If ammonium nitrate is present in water reaction will lead to formation of chloramines (NH2Cl, NHCl2, NCl3). Moreover, chlorine causes oxidation of iron (II) compounds, manganese (II), nitrates (III), sulphides and sulphates (IV) and forms aliphatic and aromatic chloro-derivatives. Chlorine present in water is toxic for living organisms. l. Chlorides Chloride is generally present in all natural surface waters, from as low concentrations as fraction of mg/dm3 to thousands of mg/dm3. Chlorides are present in natural waters due to high solubility of salts of muriatic acid (HCl) (the only exceptions are AgCl, Hg2Cl2, CuCl) and their common occurrence in the environment. The lowest concentrations of chlorides are present in rain water and in mountain streams and rivers, while the highest concentration of salts is recorded in marine waters, however high concentrations may be also found in groundwater because of naturally high levels of chloride in soils in some areas or contamination by road salt [10]. 63 WATER QUALITY CONTROL Part II. Parameters of water. Generally chlorides penetrate into natural waters from soil, natural layers of salt (natural chlorides), municipal and industrial sewage and wastes of animal origin. Chlorides are not removed in typical sewage treatment processes and they entirely pass into surface waters deteriorating their quality. In case of determination of chlorides content in water it is necessary to define their origin, if natural or pollution. In case of water pollution together with chlorides high concentration of nitrogen compounds and increase in quantity of bacteria occurs [10]. High chlorides concentration increase corrosive properties of water. Concentration above 250 mg/dm3 is harmful to vegetation [10]. There is no evidence that ingestion of chlorides is harmful to humans. Although according to sanitary-hygienic requirements chlorides content in drinking water should not exceed 250 mg/dm3, if natural chlorides. If chlorides are of different source water cannot be consumed. According to polish regulations permissible concentration of chlorides in surface waters is: I class - 250 mg/dm3 II class - 300 mg/dm3 III class - 400 mg/dm3 m. Sulphur Sulphur exists in the environment both in elemental and bonded form. However, in natural waters sulphur is present in form of dissolved hydrogen sulphide, hydrogen sulphides HS- or soluble and insoluble sulphides S2-. The form of compound depends on the water pH and presence of different ions in the solution. At the pH below 6 the main form is dissolved, not dissociated, hydrogen sulphide, whereas at pH higher than 7 hydrogen sulphides ions HSpredominates. Sulphide ions S2- occur at pH above 9 [10]. Sulphur migrates into natural waters from atmosphere, volcanic gases, industrial dusts, soil, sewage and decomposition of organic matter of plant, animal and synthetic origin. n. Sulphates Sulphates (VI) commonly occur in natural waters, contrary to sulphates which are rarely present in natural waters. They get into water due to erosion of rocks and soil, biochemical oxidation of sulphur and its compounds, atmospheric precipitation, biochemical decomposition of plant and animal proteins (in aerobic conditions) and from industrial 64 WATER QUALITY CONTROL Part II. Parameters of water. sewage. Especially high content of sulphates can be present in sewage form chemical industry. In surface waters concentration of sulphates usually vary between 10 – 60 mg/dm3. In ground waters the concentration is higher and very often exceeds 100 mg/dm3 [10]. Sulphates are one of the least toxic anions and large quantities would have to be ingested in order to health disorders to occur (especially diarrhoea type symptoms). The presence of sulphate in drinking water can result in noticeable bitter taste. Acceptable concentration of sulphates (VI) is the following: • drinking water • surface water - 200 mg/dm3 I class - 150 mg/dm3 II class - 200 mg/dm3 III class - 250 mg/dm3 o. Silica Silica does not occur in elemental form in the environment. In natural waters it usually occurs as colloidal SiO2, silica-metal compounds like Na2SiO3, Ca2SiO3, Mg2SiO3 K2SiO3 and polynuclear silicate species, such as Si4O6(OH)62-, or silicic acid H4SiO4. The sources of silica in natural waters are minerals, such as sodium feldspar albite (NaAISi3O8) present due to erosion of rocks, atmospheric precipitation and industrial sewage. Silica is present in water at normal levels of 1-30 mg/dm3. From sanitary-hygienic point of view it is not harmful to plants and animals or humans and its content is not regulated. p. Calcium Of the cations found in most fresh-water systems, calcium generally has the highest concentration. Calcium is a key element in many geochemical processes, and minerals constitute the primary sources of calcium ion in waters. Among the primary contributing minerals are gypsum, CaSO4 · 2H2O; anhydrite, CaSO4; dolomite, CaMg(CO3)2; and calcite and aragonite, which are different mineral forms of CaCO3. Calcium is present in water as a consequence of equilibrium between calcium and magnesium carbonate minerals and CO2 dissolved in water. Water containing a high level of carbon dioxide readily dissolves calcium from its carbonate minerals: 65 WATER QUALITY CONTROL Part II. Parameters of water. CaCO3 + CO2 + H2O ∆ Ca2+ + 2HCO3When the above equation is reversed and CO2 is lost from the water, calcium carbonate deposits are formed. The concentration of CO2 in water determines the extent of dissolution of calcium carbonate [3]. The content of calcium in drinking and surface waters in not regulated. World Health Organization recommends concentration of calcium in drinking water for 75 – 200 mg/dm3. q. Magnesium Magnesium, like calcium, is a compound commonly found in natural waters, and is present as Mg2+ ion. The main sources of magnesium ions are sewage inflows and minerals, such as dolomite, Mg(CO3)2 which are present as a result of soil erosion: MgCO3 + CO2 + H2O ∆ Mg2+ + 2HCO3Magnesium Mg2+ has similar properties to Ca2+ but its concentration is usually 3 to 4 times lower than Ca2+, typically 10 mg/dm3. However, with the increase in salinity the content of magnesium ions increases faster than of calcium and in marine water it can be 3 to 4 times higher. The content of magnesium in drinking and surface waters in not regulated. World Health Organization recommends concentration of magnesium in drinking water for 50–150 mg/dm3. r. Sodium The main sources of sodium in natural waters are hydrolytic decomposition of magma rocks while weathering, erosion of sedimentary rocks, as well as municipal, industrial and agricultural sewage inflows [x]. In natural waters sodium is present in form of different salts, mainly as sodium chloride (NaCl), rarely in form of sulphates (Na2SO4), salts of carbonic acid (NaHCO3, Na2CO3) or nitrates (NaNO3). All salts are well soluble in water. The presence of sodium hydrocarbons contribute to water hardness [6]. 66 WATER QUALITY CONTROL Part II. Parameters of water. The concentration of sodium ions in natural surface waters varies between a few to 30 mg/dm3. Ground waters can contain as much as 100 mg/dm3 of sodium and marine waters even 11000 mg/dm3 [10]. Sodium compounds are not removed in typical sewage treatment processes and they entirely pass into surface waters [10]. Sodium in certain amounts is an indispensable element for human organism, but higher doses can be harmful, especially for children. The content of sodium in drinking and surface waters in not regulated. World Health Organization recommends concentration of sodium in drinking water for 200 mg/dm3. s. Potassium The main sources of potassium in natural waters are hydrolytic decomposition of magma rocks due to weathering, erosion of sedimentary rocks (mineral matter, as feldspar KAlSi3O8), forest fire runoff and municipal, industrial and agricultural sewage [10]. In natural waters potassium is present in form of salts, like sodium chloride (KCl), rarely in form of sulphates (K2SO4), salts of carbonic acid (KHCO3, K2CO3) or nitrates (KNO3). All potassium salts are very well soluble in water [6]. The concentration of potassium ions in natural surface waters is much smaller than of sodium ones, despite the fact that both elements are present in lithosphere almost the same quantity and solubility of potassium salts in water is better than of sodium salts [6]. It is because K+ ions are adsorbed by soil and rocks better than Na+ and because potassium is one of the macro nutrient elements necessary for plant growth. Natural waters contain usually less than 20 mg/dm3 of potassium and marine waters about 400 mg/dm3 [10]. Potassium compounds are not removed in typical sewage treatment processes and they entirely pass into surface water. [10]. Potassium is an essential element in human nutrition and there is no limit on the amount that can be present in drinking water. World Health Organization recommends concentration of potassium in drinking water for 200 mg/dm3. t. Aluminium Aluminium ions Al3+ are present in surface waters in rather low concentrations due to weak solubility of aluminium in water. The sources of aluminium are industrial sewage inflow, 67 WATER QUALITY CONTROL Part II. Parameters of water. corrosion of aluminium tanks and water treatment process (coagulation) with the use of alum Al2(SO4)3. Aluminium salts are harmful to humans. The source of its excessive amounts can be drinking water, diet, aluminium dishes and foil and some of medicines. World Health Organization recommends concentration of aluminium in drinking water for 0.2 mg/dm3, according to Polish rules it is 0.3 mg/dm3. 2.3.3. Microbiological Parameters By far the largest health risk from water is from disease-causing (pathogenic) organisms. As the organisms are small, they are categorised within the field of microbiological parameters. Microbiological tests are for bacteria that are used as indicators for the presence of waterborne organisms that could potentially cause disease. The microbiological parameters causing the greatest risk to humans are those that come from the gut of warm blooded animals, birds and humans. For these to enter the water, contamination from human or animal excreta is required, and this needs to come from an infected person or animal. Monitoring for pathogenic organisms is complex, expensive and the laboratory testing can take days to identify the pathogens. There is an extensive range of different pathogenic organisms including bacteria, viruses or protozoa. Indicator organisms are used as a quick screening mechanism across the water supply system to determine if there is any possibility of contamination to occur. The indicators used are Coli Count, Faecal Coliforms and Total Coliforms. The presence of these indicative organisms is evidence that the water has been polluted with faeces of humans or other warm-blooded animals. These indicators are also used to test that the water has been effectively disinfected at each of the entry points of the water supply into our closed pipe system. a. Coli Count Coli Count is the smallest volume of water, expressed in cm3, in which 1 coli bacteria is present by agreement. Coli _ Count = 100 Coli _ Factor 68 WATER QUALITY CONTROL Part II. Parameters of water. Coli Factor is equal to the most probable number of coli bacteria present in 100 cm3 of water. b. Faecal Coliforms Faecal Coliforms indicate the presence of pollution from animal or human faeces. Raw sewage or untreated river water contains high levels of these bacteria. Chlorine used in the water treatment process kill these bacteria. Acceptable amount of these bacteria in water is the following: • drinking water • surface water - none I class - 1 and above II class - 0.1 and above III class - 0.1 and above 2.3.4. Parameters Concerning Substances Undesirable in Excessive Amount a. Nitrates Nitrate (NO3-) is one of the three common forms of inorganic nitrogen present in water. Nitrate occurs in water naturally in result of plant or animal material decomposition, but can also be introduced into water due to human activities, e.g. food production, where used as a preservative; use of agricultural fertilizers and manure; disposal of domestic and industrial sewage [17]. Nitrates are also present in municipal sewage after their biological treatment in aerobic conditions [10]. Extensive application of fertilizers in agriculture can cause nitrates introduction to the aquifers. Additionally, in rural areas, where high levels of nitric oxide emissions to the air are observed, this gas is converted to nitrate and then introduced to water with atmospheric precipitation. All kinds of surface waters usually contain small amounts of nitrates, less than 1mg/dm3 [6]. Because these ions are necessary for organisms to grow, seasonal changes of nitrates concentration is observed: in winter the concentration is generally higher than in summer [10]. Nitrates stimulate the growth of macrophytes and phytoplankton but simultaneously they make up for the nutrient load in water, leading to eutrophication [17]. 69 WATER QUALITY CONTROL Part II. Parameters of water. Some studies have shown there may be a relation between nitrates presence in water and gastric cancer and methemoglobinemia (which in infants is often referred to as blue baby syndrome), so a maximum guideline of 10 mg/dm3 for drinking water has been set in Poland. In case of surface waters it must not exceed [17]: I class - 1.5 mg/dm3 II class - 7 mg/dm3 III class - 15 mg/dm3 b. Nitrites Nitrite NO2- can be of organic or inorganic origin, and it is a transitional product in nitrogen cycle taking place in natural waters. Nitrite is less stable than nitrate and generally due to chemical and biochemical factors it is oxidized to nitrate or undergo reduction to ammonia in fairly short time [2]. Nitrite is manufactured as a preservative - adding nitrate to meats and fish prevents botulism. It may also be produced from excess ammonia in drinking water distribution systems that use chloramines as a disinfectant. In Poland permissible concentration of nitrates in water is not regulated. However, it is desirable that the concentration of nitrites in drinking water do not exceed 1 mg/dm3 [2]. c. Ammonia Ammonia in surface waters can be of organic origin, the product of decomposition of plant and animal matter, or of inorganic origin, formed due to chemical or biochemical reduction of nitrate and nitrite. Ammonia is an indicator of pollution originating from soil (the excessive use of ammonia rich fertilizers), atmosphere and sewage [2]. Ammonia NH3 is very unstable compound and easily undergoes nitrification: NH3 + 2O2 → NO3- + H2O + H+ or NH4+ + 2O2 → NO3- + H2O + 2H+ If buffering capacity of water is not enough to neutralize hydrogen ions made in this reaction it may lead to a decrease in water pH [6]. 70 WATER QUALITY CONTROL Part II. Parameters of water. Ammonia is present in all natural waters. Ground water and clean surface waters contain about 0.1 mg/dm3; marine water - few mg/dm3 and the concentration increases with depth. The highest concentration of ammonia is observed in waters near crude oil beds, and can be as high as 100 mg/dm3 [6]. Ammonia is toxic for aquatic organisms. Although it is a nutrient required for life, excess of ammonia can accumulate in the organism and cause alteration in metabolism or increase body pH [17]. Drinking water, according to hygienic rules, should not contain ammonia of organic origin. In case of ammonia of inorganic origin maximum acceptable concentration is equal to [2]: • drinking water • surface water - 0.5 mg/dm3 I class - 1.0 mg/dm3 II class - 3.0 mg/dm3 III class - 6.0 mg/dm3 d. Total Organic Carbon (TOC) Carbon enters the biosphere during photosynthesis: CO2 + H2O → C6H12O6 + O2 + H2O and is returned to the biosphere in cellular respiration: O2 +H2O + C6H12O6 → CO2 +H2O + energy The principle of determination of the Total Organic Carbon in water consists in combustion of organic substances at elevated temperature. Combustion results in CO2 formation which is then determined with the use of instrumental methods (usually spectrophotometric). However, before the test is done it is necessary to remove inorganic carbon from water sample. Inorganic compounds are carbonates, hydrocarbonates and dissolved carbon dioxide. To remove these compounds phosphoric acid is added. 71 WATER QUALITY CONTROL Part II. Parameters of water. e. Hydrogen sulphide Hydrogen sulphide in natural waters can be of inorganic origin and occur in the dissolved form, or of organic origin in result of anaerobic biochemical decomposition of plant and animal proteins. Hydrogen sulphide is a weak acid and dissociated in water according to the reaction [2]: H2S ∆ H+ + HSHS- ∆ H+ + S2In natural waters and in sewage hydrogen sulphide can occur as dissolved gas, or as HS- and S2- ions, depending on water pH. Non-ionised hydrogen sulphide will be present if pH below 6, while sulphite ions if pH around 10. Moreover, sulphites can exist in anaerobic conditions, otherwise they oxidise to sulphur and sulphates [2]. The main sources of hydrogen sulphide in water include sewage and natural processes of decomposition - sulphur-reducing bacteria are the primary producers of large quantities of hydrogen sulphide. These bacteria chemically change natural sulphates in water to hydrogen sulphide. Sulphur-reducing bacteria live in oxygen-deficient (anaerobic) conditions such as deep wells, plumbing systems, water softeners and water heaters [15]. Hydrogen sulphide in ground waters can be of geochemical origin (minerals) [10]. Water containing hydrogen sulphide usually does not pose a health risk, but it does give water a nuisance "rotten egg" smell and taste. The minimum concentration detectable by taste in drinking waters is 0.05 mg/dm3 [15]. Hydrogen sulphide’s presence in drinking water when released in confined areas has been known to cause nausea, illness and, in extreme cases, death. Water supplies with as little as 1.0 mg/dm3 hydrogen sulphide are corrosive, may tarnish copper and silverware, and occasionally release a black material that stains laundry and porcelain [16]. Drinking water must not contain any hydrogen sulphite, regardless of the origin, organic or inorganic. f. Iron The main source of iron in natural waters is erosion of minerals from rocks and soil. It is also introduced to water with acid mine, sewage from metallurgical, dyeing and galvanizing plants and due to corrosion of pipelines and steal constructions [2]. 72 WATER QUALITY CONTROL Part II. Parameters of water. Iron is present in water as Fe(II) and Fe(III) in dissolved, colloidal or suspended form. In presence of oxygen or oxidizing agents Fe(II) compounds easily oxidize to Fe(III) ones, which precipitate as hydroxides or oxides. Colloids can appear if organic substances are present in water, e.g. humic substances [2]. Iron is an essential element in human nutrition (blood formation). Large quantities of iron in drinking water (acceptable 0.5 mg/dm3) cause turbidity, yellowish colour and an unpleasant taste. Concentrations for surface waters are [2]: I class - 1.0 mg/dm3 II class - 1.5 mg/dm3 III class - 2.0 mg/dm3 g. Manganese Manganese in the environment occurs in form of Mn2+ and Mn4+. The original source of manganese compounds in natural waters is weathering of magma rocks and sedimentary rocks. Manganese can also get into surface waters with industrial sewage, atmospheric dust and decomposition of plant material. Concentration of manganese ions in ground waters is generally higher than in surface waters and varies between 0.1 to 0.4 mg/dm3. This is because of good oxidation and higher pH of surface water which leads to precipitation of insoluble MnO2 • H2O and its further sedimentation [10]. Manganese causes water to cloud up. It is connected with precipitation of insoluble sediments. It also causes undesirable taste of water and is conductive to bacterial growth. At levels above 0.15 mg/dm3, manganese stains plumbing fixtures and laundry [10]. Manganese is an essential component of living organisms, both plant and animal ones, as it catalyses biochemical reactions [x]. However, in large dose it is toxic to organisms [2]. Acceptable concentration of manganese in water in Poland is set to be not higher than [2]: • drinking water • surface water - 0.1 mg/dm3 I class - 0.1 mg/dm3 II class - 0.3 mg/dm3 III class - 0.8 mg/dm3 h. Copper Copper is widely characterised in chapter 3 “Human Impact on Water Resources”, point 3.4. 73 WATER QUALITY CONTROL Part II. Parameters of water. Permissible concentration of copper in drinking water Poland is 0.05 mg/dm3 or less, because this level is below our taste threshold but contributes to minimum nutritional requirements [6]. In case of surface waters it is [2]: I class - 0.01 mg/dm3 II class - 0.1 mg/dm3 III class - 0.2 mg/dm3 i. Phosphorus Phosphorous occurs in natural waters as anions of orthophosphoric acid H3PO4. The anions H2PO4- and HPO42- are predominant in normal water pH ranges. It may also be present as organic phosphorus [3]. Phosphorous is delivered to surface waters with fertilizer runoff form agricultural human activity, erosion of rocks (also mining), with domestic wastes due to use of detergents, industrial wastes and as a result of decay of organic matter (plants and animals origin)[2]. Phosphorous is an algal nutrient often contributing to excessive algal growth and eutrophication [3]. Phosphates are sometimes added to drinking water to reduce corrosion and precipitation of certain compounds. It is not harmful in clean waters. Acceptable concentration of phosphates in surface waters is [2]: I class - 0.2 mg/dm3 II class - 0.5 mg/dm3 III class - 1.0 mg/dm3 j. Fluoride Fluoride is present in minerals, soils, and is naturally found in different concentrations in natural waters [19]. It can get into waters also with industrial sewage, agricultural runoff and due to burning of coal. It forms HF at low pH and in the presence of Ca(II), Ba(II), Sr(II) and Pb(II) forms insoluble salts. Concentration of fluorides in surface waters is usually less than 1 mg/dm3 [10]. The presence of small quantities of fluoride in drinking water leads to a substantial reduction of dental cavities, particularly among children (at levels around 1 mg/dm3), and is commonly added to water for that purpose. However fluoride is harmful to bones and teeth in concentration above approximately 10 mg/dm3 [3]. According to WHO optimum concentration of fluoride in drinking water is from 0.9 to 1.7 mg/dm3 (depending on temperature). For middle Europe this concentration varies between 0.8 74 WATER QUALITY CONTROL Part II. Parameters of water. to 1.5 mg/dm3 [6]. In Poland it is between 0.3 to 1.5 mg/dm3. Permissible concentrations in surface waters should equal to: I and II class - 1.2 mg/dm3 III class - 2.0 mg/dm3 k. Dissolved or emulsified hydrocarbons - mineral oils Crude oil is a complex mixture of hydrocarbons (containing up to 90 carbon atoms in a molecule [8]) with minor proportions of other chemicals such as compounds of sulphur, nitrogen and oxygen. To use the different parts of the mixture they must be separated from each other. This separation is called refining [16]. Refining is carried out in different temperature ranges. Mineral oils are obtained from crude oil as a fraction collected between 330 and 390°C. It is a mixture of nonpolar aliphatic hydrocarbons containing 15 to 22 carbon atoms in a molecule [8]. Mineral oils occur in natural waters mainly due to inflow of municipal and industrial sewage (industries using or producing oils), runoff from roads and municipal areas. Also ships and motorboats can be significant sources of water pollution with oil [8]. Crude oil and its products of refining are practically insoluble in water. However, in presence of emulgents (e.g. surfactants) emulsions form easily [10]. Oils present in water very often flow on the water surface as a thin layer, showing pollution of water reservoir [8]. According to polish regulations for all classes of water oils must not be visible on the surface of water. l. Phenols Phenols are benzene derivatives in which hydroxide groups –OH are bounded directly to carbon atoms in aromatic ring [10]. Some typical phenol contaminants are the following [3]: OH CH3 CH3 CH3 OH OH OH OH OH OH OH phenol o-cresol m-cresol p-cresol l-naphtol hydrochinon chlorophenol 75 WATER QUALITY CONTROL Part II. Parameters of water. Phenols in natural waters occur in trace concentration, but in case of industrial pollution the concentration can be as high as few mg/dm3 [8]. They are formed while biochemical decomposition of plant material and humic substances in bottom sediments. Some amounts of phenols get into waters with sewage from coking plants, gas-works and petrochemical plants [10]. Surface waters, contaminated with phenols, after chlorination develop disgusting taste and odour due to formation of chlorophenols and due to oxidation of phenols to chinons, e.g. [10]: Phenols are easily biodegradable substances unless they are in concentrations toxic for microorganisms [8]. The most simple and common compound from the phenolic group is phenol [8]. Phenol is a toxic substance causing denaturation of proteins [10]. It is a protoplasmic poison that damages all kinds of cells and is alleged to have caused “an astonishing number of poisonings” since it came into general use (on wounds and in surgery). The acute toxicological effects of phenol are predominantly upon the central nervous system and death can occur as soon as one-half hour after exposure. Acute poisoning by phenol can cause severe gastrointestinal disturbances, kidney malfunction, circulatory system failure, lung oedema and convulsions. 76 WATER QUALITY CONTROL Part II. Parameters of water. Fatal doses of phenol can be absorbed through the skin, by respiration and alimentary canal [3]. Polish regulations concerning drinking water define that the smell of phenols should be undetectable. m. Surfactants (Surface Active Agents) The surface-active agent is the principal component of a synthetic detergent but is also assisted in its cleaning agent role by the builders used (sometimes called built detergents). The surface-active agents are compounds that have two groups present in the molecule: one being hydrophobic in nature and one being hydrophilic (water-liking) in nature [8]. Surfactants are harmful for aquatic environment (fish, plankton, plants) and in higher concentrations for humans as well. They cause formation of abundant foam which makes oxygen diffusion difficult and inhibits self-purification of water. Surfactants in surface waters act as emulgents facilitating formation of oil emulsions. A part of surfactants containing phosphates or polyphosphates contribute to eutrophication. n. Trihalomethanes (THMs) Trihalomethanes (THMs) are a group of four chemicals that are formed when chlorine, used to microbial contaminants control in drinking water, react with organic matter naturally occurring in water. More detailed characteristics is included in chapter 4 “Water for Different Purposes”, point 4.7.4. The Environmental Protection Agency has set a Maximum Contaminant Level (MCL) of 100 µg/dm3 for TTHM (total trihalomethanes) in drinking water. The new by-products rule cut this down to 70 µg/dm3 and will reduce this even further to 40 µg/dm3. 2.3.5. Parameters Concerning Toxic Substances The term "toxic" refers to the ability of a physical, biological or chemical agent to provoke an adverse effect or deleterious response in an organism. The term does not include amount or dose of the substance required to elicit the adverse effect. A toxic substance is a chemical pollutant that is not a naturally occurring substance in aquatic ecosystem and above a certain level of exposure or dose has detrimental effects on tissues, organs, or biological processes. Toxicants in water and sediments are mainly heavy metals, 77 WATER QUALITY CONTROL Part II. Parameters of water. persistent organic pollutants (POPs) and radioactive compounds. Waters affected by those toxicants can have serious influence on the aquatic ecosystems and can make water unsuitable for human consumption. Lethal dose (LD) is the amount of a substance (in mg per kg of mass of experimental organism) or physical agent (radiation) that causes death when taken into the body by a single absorption [21]. LD50 is an abbreviation for “Lethal Dose 50%.” It is sometimes also referred to as the “Median Lethal Dose”. The LD50 for a particular substance is essentially the amount (in mg per kg of mass of experimental organism) that can be expected to cause death in half (50%) of a group of some particular animal species, usually rats or mice, when entering the animal’s body by a particular route [27]. The LC(t)50 (lethal concentration 50% for exposure time t) is a similar and widely used measure for acute toxicity by inhalation. The LC(t)50 is essentially the concentration of a substance that can be expected to cause death in half of a group of some particular species when entering the body over the specified period of time [27]. In Poland there is a division for 5 classes of toxicology (Monitor Polski, 1965, Nr 28, poz. 156) and they are shown in Table 2.6. Table 2.6. Division of toxicology classes Class of toxicology LD50 for a rat [ in mg/kg ] I to 50 II 51-150 III 151-500 IV 501-5000 V Above 5000 Level of threat Highly poisonous (toxic) Poisonous Very harmful substances Harmful substances Practically harmless Parameter describing a toxic substance is also persistency. It is explained in chapter 3 “Human Impact on Water Resouces”, point 3.1.1. Although both natural and synthetic chemicals may cause a variety of toxic effects at high enough doses, the effect of most concern is cancer. Cancer is a disease characterized by rapid growth of cells in the body, often in the form of a tumour. Cancer is invasive - that is, it can spread to surrounding tissues. Although this disease is a leading cause of death in many countries around the world, research has provided considerable insight into its many causes. These may include external, environmental factors comprising 80-90% of the causes of cancer, such as carcinogenic substances, exposure to 78 WATER QUALITY CONTROL Part II. Parameters of water. irradiation, and, viruses and internal factors, the instances in lowering of immune functions caused by heredity, agedness and change in lifestyle. According to a report from World Health Organization (WHO), 35% of carcinogenic substances are derived from food and drinks, and 30% are from smoking as the second rank [28]. Chemicals which are known to cause cancer are called carcinogens and are divided into 5 groups, listed in Table 2.7. Table 2.7. Division for groups of carcinogenicity. Group of carcinogenicity 1 2A 2B 3 4 Characteristics The agent is carcinogenic to humans; Used only when there is sufficient evidence of carcinogenicity in humans, eg. Arsenic The agent is probably carcinogenic to humans; Used only when there is limited evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals, eg. PCBs The agent is possibly carcinogenic to humans; Used only when there is limited evidence of carcinogenicity in humans and insufficient evidence of carcinogenicity in experimental animals, eg. TCDD The agent is not classifiable as to its carcinogenicity to humans; If the evaluated data is insufficient to allow classification into any other group, eg. Cholesterol The agent is probably not carcinogenic to humans; An agent exhibits evidence suggesting a lack of carcinogenicity in humans and in experimental animals, eg. caprolactam The strongest evidence that a compound is a carcinogenic hazard for man is epidemiological, although most known human carcinogens are found to be carcinogenic for experimental animals. There is no evidence that all substances which are carcinogenic for animals are also carcinogenic for man, but it is difficult to declare any compounds as being non-carcinogenic for man when it has been shown to be carcinogenic in animal studies. Up to now, scientists have identified about two dozen chemicals or occupational exposures which appear to be definitely carcinogenic to humans. In addition, there are a number of chemicals which cause cancer in animals and are suspected of being human carcinogens. It must be remembered, however, that as with all toxic effects, the dose or amount of exposure is critical [29]. Heavy metals presented below (arsenic, cadmium, chromium, lead and mercury) are precisely characterised in chapter 3 “Human Impact on Water Resources”, point 3.4. This chapter focuses mainly on some toxic effects caused by drinking water contamination with particular heavy metal, and permissible concentrations of metals in drinking and surface waters. 79 WATER QUALITY CONTROL Part II. Parameters of water. a. Arsenic Even very low concentrations of arsenic in drinking water appear to be associated with a higher incidence of lung or bladder cancer [3]. According to WHO arsenic content in drinking water should not exceed 0.05 mg/dm3. Polish law sets the maximum concentration of arsenic in drinking water also for 0.05 mg/dm3, and for surface waters: I and II class - 0.05 mg/dm3 II class - 0.05 mg/dm3 III class - 0.2 mg/dm3 b. Cadmium In drinking water maximum acceptable concentration of cadmium is 0.005 mg/dm3. According to polish regulations permissible concentration in surface waters is [6]: I class - 0.005 mg/dm3 II class - 0.03 mg/dm3 III class - 0.1 mg/dm3 c. Chromium The Environmental Protection Agency (EPA) established Maximum Contaminant Level (MCL) to 0.1 mg/dm3 as total chromium [19]. Polish regulations set the value of 0.01 mg/dm3 of chromium (VI) in drinking water. In surface waters maximum permissible concentration of chromium (VI) is equal to 0.05 mg/dm3 in all classes of waters, and in case of chromium (III) it is: I class - 0.05 mg/dm3 II class - 0.1 mg/dm3 III class - 0.1 mg/dm3 d. Lead Excess quantities of lead may impact human health, especially affecting small children. Therefore a very conservative limit has been set at 0.05 mg/dm3 of lead in drinking water (according to WHO) [6]. In Poland the value for drinking water is exactly the same, while in case of surface waters it is equal to 0.1 mg/dm3 in all classes of water. 80 WATER QUALITY CONTROL Part II. Parameters of water. e. Mercury A very conservative guideline of 0.001 g/m3 of mercury in drinking water has been established in Poland on the basis of health considerations. In surface waters the maximum permissible concentration is: I class - 0.001 mg/dm3 II class - 0.005 mg/dm3 III class - 0.01 mg/dm3 f. Cyanides Cyanide is a deadly poisonous substance which exists in water as HCN, a very weak acid [19]. Large quantities of cyanide are used in industry, especially for metal cleaning and electroplating. It is also one of the main gas and coke scrubber effluent pollutant form gas works and coke ovens. Cyanide is widely used as a pesticidal fumigant and in certain metalprocessing operations. The presence of cyanide in water is indicative a serious pollution problem [3]. According to Polish regulations permissible concentration is equal to 0.01 g/m3. g. Selenium Selenium naturally occurs in the earth's crust and is commonly found in sedimentary rock. Much of the selenium in rocks is combined with sulfide minerals or with silver, copper, lead, and nickel minerals. Selenium can exist naturally: • In inorganic forms at different oxidation states: -II (selenide), 0 (elemental selenium), +IV (selenite SeO32-), +VI (selenate SeO42-); • In the form of organic compound and methylated derivatives; each form differing widely in their nutritional and toxic impact. Each form differs widely in their nutritional and toxic impact. The anthropogenic sources of selenium include sewage and wastes disposal [6]. In soil and water selenium exists mainly in form of inorganic ions: Se (IV) and Se (VI). Total Se levels in environmental samples range from about 0.1 - 400 µg/dm3 in natural waters to 0 80 ng/kg in soils. Selenium transits into organic linkages under biomethylation process, as a result of microorganisms and plants activity. Well known in our environment organic species of selenium are associated to aminoacids (e.g. selenocystamine SeCM, selenocystine SeCYS, 81 WATER QUALITY CONTROL Part II. Parameters of water. selenomethionine SeM). The less toxic forms seem to be volatile methylated selenium compounds (e.g. dimethylselenide DMSe, dimethyldiselenide DMDSe), which are metabolised after detoxification processes. In small amounts selenium is a prerequisite for humans. Sufficient selenium supplementation can protect against heart disease. Se deficiency can cause many diseases, such as hemolysis, multiple sclerosis and rheumatic arthritis, and is most critical for the brain and infant growth. However, transition from required level to toxic dose is quite easy. Detoxification effects of Se by interaction with other metals are proven and widely described; the toxicity of Se is modified by complexation with As, Ag, Cu, Hg. In real life the toxicity of selenium against environment and human being strongly depends on the specific form. Maximum permissible concentration of selenium in drinking water is 0.01 mg/dm3 (WHO regulations) and also in Poland is equal to 0.01 mg/dm3 [6]. In case of surface waters it is equal to 0.01 mg/dm3 in all classes of water. h. Radioactive compounds Radioactivity is defined as spontaneous nuclear transformation of nuclide into another nuclide, accompanied by emission of nuclear radiation, either corpuscular of electromagnetic [9]. Radioactivity of nuclides naturally occurring in the environment is called natural activity, while radioactivity of nuclides obtained in nuclear reactions is called artificial radioactivity. Radioactive elements are spread in rocks, form which they pass into waters. They can also get into waters with atmospheric precipitation and inflow of radioactive sewage. Natural radioactivity is caused by atmosphere – 3H and 14 90 Y, isotopes: Sr, 89 Sr, 90 226 Ra, 222 Ru, 238 U, 230Th 210 , Pb, 40 K and isotopes from the C. Acquired radioactivity caused by water pollution with radioactive 91 Y, 131 I, 132 I, 137 Cs, 141 Cs, 144 Ce, 32 P [6]. Radio-isotopes the most commonly found in sewage are: 24Na, 32P, 40K, 60Co, 85Zn, 90Sr, 131I and 137Cs [2]. Naturally radioactive waters are divided into two groups: • Waters containing only radon and its emanation – these are radon-waters; most of health waters are radon-waters; • Radium waters with high radium content – these are mainly deep-waters [6]. Radionuclides can be present in waters in form of dissolved ions or complexes, or insoluble form, depending on pH, redox potential and composition of water [6]. Radionuclides present in waters are absorbed by aquatic organisms, which directly or along the food chain enter humans [2]. The most common isotopes present in water are radon, 82 WATER QUALITY CONTROL Part II. Parameters of water. uranium and radium. Their concentration varies between 0.1 to 50 mg/m3. Waters containing below 10-7 g Ra in m3 are numbered among weakly radioactive, between 10-7 and 10-6 of average radioactivity, and above 10-6 g/m3 highly radioactive. Artificial radioactivity originating from human activities (extraction and enrichment of uranium, exploitation of nuclear reactors) is very dangerous for humans, animals and plants. The most toxic isotopes are: 90Sr, 90Y, 210Pb, 210Po, 226Ra, 238U. Their maximum concentration in drinking water must no excess 10-4 µCi/cm3 [6] 4. In order to protect people against extremely harmful radioactive radiation continuous monitoring of the level of water and sewage radioactivity is necessary [2]. Radioactivity and radioactive pollution are also described in chapter 3 “Human Impact on Water Resources”, point 3.8. i. Persistent Organic Pollutants (POPs) Persistent Organic Pollutants (POPs) are precisely described in chapter 3 “Human Impact on Water Resources”, point 3.7. Table 2.8 contains only concise and general information on POPs. Table 2.8. Summary of Persistent Organic Pollutants properties. Type of pollutant PAHs PCBs Sources in natural waters Industrial sewage; leaky Compounds built up of tanks with few aromatic rings; crude oil, practically insoluble in fuels; dusts water, can form and sooth suspensions; pile up in falling onto sediments [10]. the ground (runoff); roads surface [10]. General characteristics PCBs include about 200 different compounds, in water environment 60 were determined [8]. Sewage; surface runoff; rain [8]. Concentration in natural waters little polluted waters 50 – 250 ng/dm3; medium polluted waters 250 – 1000 ng/dm3; highly polluted waters above 1000 ng/dm3 [8]. Usually between 0.01 to 10 ng/dm3 [8]. Surface runoff Used for annihilation from agricultural of pests; divided for Between 0.001 and Pesticides three groups: areas; 2.8 µg/dm3 insecticides, herbicides, municipal and industrial fungicides [10]. sewage [10]. Acceptable concentration in drinking water Maximum concentration of PAHs, according Carcinogenic; to WHO [8] regulations 0.02 bioaccumulate µg/dm3. in aquatic Maximum organisms [10]. concentration of benzo(a)pyrene 0.015 µg/dm3 [10]. Harmful to Maximum aquatic environment; concentration of bioaccumulate pentachlorophenol 0.01 mg/dm3 [10]. in aquatic organisms [8]. Toxic effect Bioaccumulate in living organisms; toxic for humans [10]. Maximum concentration of heptachlor 0.0001 mg/dm3 [10]. 4 1 Ci (CURIE) – obsolete unit of radioactivity giving 3.7•1010 disintegrations per second; in SI system 1 Ci = 37 GBq 83 WATER QUALITY CONTROL Part II. Parameters of water. Dioxins By products of: herbicide production; municipal and hospital wastes combustion; [10] Can contain between 1 and 8 chloride atoms in a molecule; practically insoluble in water. Industrial sewage Depends on the amount of chlorine atoms; Toxic dioxins are those containing 4,5 and 6 atoms of chlorine; skin irritation; liver damage; carcinogenic and mutagenic Maximum concentration of DDT 0.001 mg/dm3 [10]. References: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. E.L. Katz “The stability of turbidity in raw water and its relationship to chlorine demand”, Journal of the American Water Works Association, 1986 K. Lipkowaska – Grabowska, E. Faron – Lewandowska “Pracownia chemiczna. Analiza wody i ścieków”, Warszawa 1998 Stanley E. Manahan “Fundamentals of Environmental Chemistry”, Lewis Publishers, USA 1993 A.G.Howard “Aquatic Environmental Chemistry”, Oxford University Press, Oxford 1998 M.W. LeChevallier, T.M. Evans, R.J. Seidler “Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Applied and environmental microbiology”, 1981 W.F. McCoy, B.H. Olson “Relationship among turbidity, particle counts and bacteriological quality within water distribution lines”, 1986 Department of National Health and Welfare (Canada) “Guidelines for Canadian drinking water quality”, 1991 J.R. Dojlido „Zanieczyszczenia wód” Dictionary of chemical terminology, edited by Dobromiła Kryt, Wydawnictwa Naukowo-Techniczne, Warszawa 1980 [x] [x2] [x3] [x6] http://www.aldeaglobal.com.ar/agua/wqsi_taste.htm On the basis of the following publications: a. L.M.Bartoshuk “NaCl thresholds in man: thresholds for water taste or NaCl taste?”, Journal of comparative physiology, 1974 b. Water Research Centre “A guide to solving water quality problems in distribution systems”, Medmenham, 1981 c. J.M. Cohen „Taste threshold concentration of metals in drinking water”, Journal of the American Water Works Association, 1960, 52(5):660-670 d. E.J. Thurmann “Organic geochemistry of natural waters”, Amsterdam, Netherlands, Martinus Nijhoff 1985 e. K.M. MacKenthun, L.E.Keup “Biological problems encountered in water supplies”, Journal of the American Water Works Association, 1970, 62(8):520-526 f. J.E. Wajon “The occurrence and control of swampy odour in the water supply of Perth, Western Australia”, Bentley, Western Australia, School of Applied Chemistry, Western Australia Institute of Technology, 1985 g. F.B. Whitfield, D. Freeman “Off-flavours in crustaceans caught in Australian coastal water”, Water science and technology, 1983, 15(6/7):85-95. h. A.C. Linden, G.J.E. Thijsse “The mechanism of microbial oxidation of petroleum hydrocarbons”, Advances in enzymology, 1965, 27:469-546. i. W.H. Glaze “Reaction products of ozone: a review”, Environmental health perspectives, 1986, 69:151. 84 WATER QUALITY CONTROL Part II. Parameters of water. j. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. C. Anselme “Removal of tastes and odours by the ozone-granular activated carbon water treatment processes”, Paper presented at the 7th Ozone World Congress, International Ozone Association, Tokyo, 1985 k. I.H. Suffet “Removal of tastes and odours by ozonation”, Proceedings of the American Water Works Association Annual Conference, Seminar on Ozonation. Denver, CO, AWWA, 1986 l. R.H. Burttschell “Chlorine derivatives of phenol causing taste and odour”, Journal of the American Water Works Association, 1959, 51(2):205-214 http://www.ianr.unl.edu/pubs/water/g1275.htm http://ohioline.osu.edu/lines/search.html http://www.aaawatertesting.com/nitrogen.htm#EPA http://www.eco-usa.net/toxics/index.shtml http://www.clwa.org/chromium.htm http://water.nr.state.ky.us/ww/ramp/rmalk.htm http://www.nrc.gov/reading-rm/basic-ref/glossary/lethal-dose.html http://www.rpi.edu/dept/chem-eng/Biotech-Environ/Adsorb/adsorb.htm http://ias.vub.ac.be/General/Adsorption.html http://www.deq.state.mi.us/documents/deq-swq-npdes-DissolvedOxygen.pdf http://www.deq.state.mi.us/documents/deq-swq-npdes-BiochemicalOxygenDemand.pdf http://www.chemind.fi/english/education/index.html http://www.ilpi.com/msds/ref/ld50.html http://www.aboutcancer.info/Food/food.html http://www.iet.msu.edu/Tox_for_Public/carcin.htm 85