Practice Problems 2
advertisement
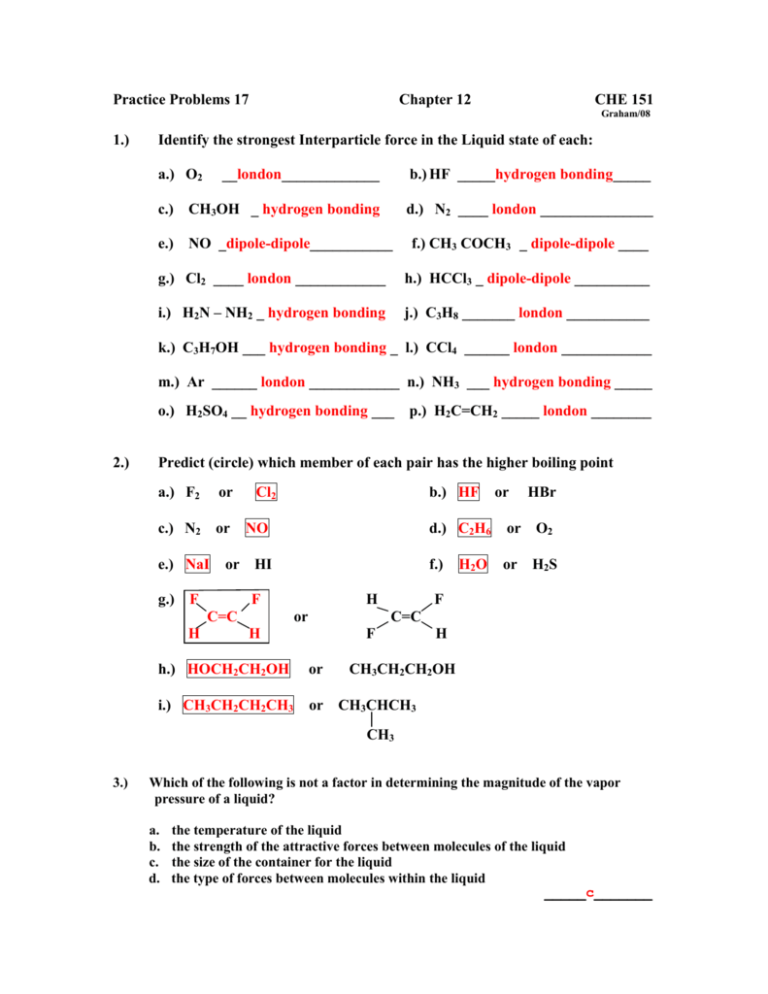
Practice Problems 17 Chapter 12 CHE 151 Graham/08 1.) Identify the strongest Interparticle force in the Liquid state of each: a.) O2 __london_____________ b.) HF _____hydrogen bonding_____ c.) CH3OH _ hydrogen bonding d.) N2 ____ london _______________ e.) NO _dipole-dipole___________ f.) CH3 COCH3 _ dipole-dipole ____ g.) Cl2 ____ london ____________ h.) HCCl3 _ dipole-dipole __________ i.) H2N – NH2 _ hydrogen bonding j.) C3H8 _______ london ___________ k.) C3H7OH ___ hydrogen bonding _ l.) CCl4 ______ london ____________ m.) Ar ______ london ____________ n.) NH3 ___ hydrogen bonding _____ o.) H2SO4 __ hydrogen bonding ___ 2.) p.) H2C=CH2 _____ london ________ Predict (circle) which member of each pair has the higher boiling point a.) F2 or c.) N2 or e.) NaI Cl2 NO or HI g.) F F C=C H b.) HF H or or HBr d.) C2H6 or O2 f.) or H2S H2 O F C=C H F h.) HOCH2CH2OH or i.) CH3CH2CH2CH3 or CH3CHCH3 H CH3CH2CH2OH CH3 3.) Which of the following is not a factor in determining the magnitude of the vapor pressure of a liquid? a. b. c. d. the temperature of the liquid the strength of the attractive forces between molecules of the liquid the size of the container for the liquid the type of forces between molecules within the liquid _____c_______ 4.) A nonvolatile liquid would a. b. c. d. e. 5.) have weak attractive forces between molecules evaporate rapidly at room temperature have a high vapor pressure at room temperature be a very "explosive" substance have strong attractive forces between molecules _____e_________ Which statement about the boiling point of water is incorrect? The boiling point is greater than 100oC in a pressure cooker. The boiling point is less than 100oC for locations at low elevations. At sea level and at a pressure of 760 mm Hg, the boiling point is 100oC. In a pressure cooker, shorter cooking times are required due to the change in boiling point. d. The boiling point is a consequence of a molecule's intermolecular forces. a. b. c. d. ____b__________ 6.) Which of the following statements is correct? a. b. c. d. dipole-dipole interactions occur only between nonpolar molecules a hydrogen bond is an extremely weak dipole-dipole interaction London forces are "instantaneous" dipole-dipole interactions Hydrogen bonding occurs between any two hydrogen-containing molecules ____c__________ 7.) Which of the following substances would be expected to have the lowest boiling point? a. b. c. d. e. 8.) a nonpolar liquid a polar liquid with hydrogen bonding a polar liquid with weak dipole-dipole interactions a nonvolatile liquid a polar liquid with strong dipole-dipole interactions ____a_________ Which of the following compounds is incorrectly matched with the predominant intermolecular force associated with that compound in the liquid state. compound a. CCl4 b. H2O c. BF3 d. NH3 e. Br2 intermolecular force dipole-dipole interactions hydrogen bonding London forces hydrogen bonding London forces ____a________ 9.) The normal boiling point of a substance is determined by its molecular mass and its intermolecular forces. Considering these two factors, predict the order of increasing boiling points for the following substances: H2, NaCl, H2O, and CO2. a. b. c. d. e. 10.) 11.) CO2 < H2 < NaCl < H2O H2 < CO2 < H2O < NaCl H2O < NaCl < CO2 < H2 H2 < NaCl < H2O < CO2 NaCl < H2O < CO2 < H2 ____b________ The vapor pressure of PBr3 reaches 400 mm Hg at 150oC. The vapor pressure of PCl3 reaches 400 mm Hg at 57oC. a. At 100oC which substance should evaporate at the faster rate? ____PCl3_____ b. Which substance should have the lower normal boiling point? ____ PCl3____ c. Which substance should have the weaker intermolecular forces? ____ PCl3___ d. At 50oC which substance should have the higher vapor pressure? ____ PCl3____ Consider the phase diagram shown below. a. What phase(s) is/are present at point A? ___liquid_____________________ b. What phase(s) is/are present at point B? _solid, liquid, gas_______________ c. Name point C and explain its significance. _critical point- above the critical temp., substance cannot be liquefied, regardless of pressure._________________ d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen. D = solid phase. As P is lowered, solidgas dividing line is reached. Solid will sublime. ___________ 12.) A. B. C. D. E. 13.) Which of the following atoms should have the greatest polarizability? F Br Po Pb He ____D________ In which of the following compounds will the molecules not form hydrogen bonds with each other? _____c______ 14.) A. B. C. D. E. 15.) A. B. C. D. E. Which of the following pairs is arranged with the particle of higher polarizability listed first? Se2-, S2I, IMg2+, Mg Br, I None of these choices is correct ____A_______ Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first. C7H16, C5H12 CCl4, CBr4 H2O, H2S CH3CH2OH, CH3-O-CH3 Xe, Kr _____B_______ 16.) Which of the following should have the highest surface tension at a given temperature? A. B. C. D. E. 17.) CH4 CF4 CCl4 CBr4 CI4 _____E________ Which of the following should have the highest surface tension at a given temperature? _____d_____ 18.) When the adhesive forces between a liquid and the walls of a capillary tube are greater than the cohesive forces within the liquid A. the liquid level in a capillary tube will rise above the surrounding liquid and the surface in the capillary tube will have a convex meniscus. the liquid level in a capillary tube will rise above the surrounding liquid and the surface in the capillary tube will have a concave meniscus. the liquid level in a capillary tube will drop below the surrounding liquid and the surface in the capillary tube will have a convex meniscus. the liquid level in a capillary tube will drop below the surrounding liquid and the surface in the capillary tube will have a concave meniscus. None of these will occur. B. C. D. E. _____B_________ 19.) Which of the following factors contributes to a low viscosity for a liquid? A. B. C. D. E. low temperature spherical molecular shape hydrogen bonding high molecular weight high boiling point ____B_______ 20.) Which of the following pairs of substances is arranged so that the one with higher viscosity is listed first? ____b______ 21.) Which of the following liquid substances would you expect to have the lowest surface tension? A. B. C. D. E. Pb CH3OCH3 HOCH2CH2OH H2O CH3CH2OH ____B_______ 22.) Which one of the following substances does not exist in the indicated solid type? A. B. C. D. E. graphite - network Na - metallic SiO2 - molecular NaCl - ionic diamond – network _____C______ 23.) When liquid bromine is cooled to form a solid, which of the following types of solid would it form? A. B. C. D. E. atomic metallic molecular ionic covalent network _____C______ 24.) A. B. C. D. E. 25.) For the solid forms of the following elements, which one is most likely to be of the molecular type? Xe C Pb S Cr _____D_____ Of the five major types of crystalline solid, which would you expect each of The following to form? (e.g., H2O: molecular) A. Sn ____metallic__________ B. Si ____network covalent__ C. KCl ____ionic____________ D. Xe _____atomic__________ E. F2 ____molecular_________









