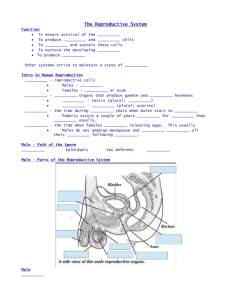
Relationship between Mineral Composition of Seminal
advertisement

R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. Acta Scientiae Veterinariae, 2012. 40(2): 1027. ORIGINAL ARTICLE Pub. 1027 ISSN 1679-9216 (Online) Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds Reza Asadpour ABSTRACT Background: Seminal plasma of mammals is a complex fluid, which serves as a carrier for the spermatozoa on their journey from the male testes to their target, the oocyte. Seminal plasma contains a variety of biochemical components, some of which are relatively specific for the regulation of sperm function. The objective of this study was to investigate enzyme activities aspartate-amino-transferase (AST), alkaline phosphatase (ALP), lactate-dehydrogenase (LDH) and concentrations of macroelements[ sodium (Na+) and potassium (K+) and calcium (Ca2+) ] in seminal plasma. Moreover the present study was performed to investigate proteins in ram seminal plasma and the correlation between specific proteins and semen characteristics in cross breed ram. Materials, Methods & Results: Sixteen crossbred fertile rams (four Baluchi × Moghani, four Ghezel × Baluchi, four Ghezel × Merino, and four Merino × Moghani) were used in this study. Three ejaculates of each animal were collected during the breeding season and examined spermatozoa with light microscopy for motility, concentration, for dead sperm and morphology. Seminal plasma was harvested by centrifugation and then subjected to analysis enzyme, mineral composition and SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Total protein, AST, ALP, LDH, Na+, K+ and calcium Ca2+ were measured. Spearman’s correlation coefficient test was applied to examine the correlation between seminal plasma protein fractions with all the parameters of the semen. Differences were considered to be statistically significant at P < 0.05. The highest seminal plasma concentration of Na+, K+ and Ca2+ was recorded in Ghezel ×Merinos rams. The result showed that there was a significant negative correlation between Na+ and K+ but small correlation was recorded between Na+ and Ca2+. The highest plasma concentration of ALP was recorded in Baluchi × Moghani ram and the lowest was recorded N in Merino × Moghani ram (8 ± 1.25 and 3.33 ± 0.01 U/mL, respectively). The highest plasma concentration of LDH and AST was recorded in Ghezel × Baluchi ram (4.68 ± 0.19 U/mL, 19 ± 4 U/mL respectively). Significant correlations were found between LDH levels and sperm viability. The results obtained indicate that ram seminal plasma protein profile is characterized by many protein bands with molecular weights, ranging from 8.81 to 95.82 kDa. Five proteins in this study had quantitative difference between seminal plasma from crossbred rams. Discussion: The results showed that the relative content of protein bands wasn’t significantly different by semen characteristics. Thus this study indicates that the relative content of seminal plasma proteins could not be an essential index to evaluate ram semen quality. Also increase in the percentage of live and normal sperm corresponded to an increase in LDH activity in the seminal fluid. It has been stated that LDH plays an important metabolic role in sperm capacitation and fertilization. This study suggest that the cations Na+ and K+ generally establish the osmotic balance, and seminal plasma osmolality ultimately plays an important role in the activation sperm cell also potassium ions (K+) are also intracellular cations, and the concentrations in the seminal fluid may be an indication of sperm plasma membrane integrity. Thus some enzyme and mineral composition could be an essential index to evaluate cross breed ram semen quality. Keywords: minerals, rams, SDS-PAGE, seminal plasma, semen quality. Received: September 2011 www.ufrgs.br/actavet Accepted: December 2011 Department of Clinical Science, Faculty of Veterinary Medicine, University of Tabriz CORRESPONDENCE: R. Asadpour [r_asadpour@tabrizu.ac.ir Fax: +98 (411) 3357834]. Faculty of Veterinary Medicine, University of Tabriz. PK: 51666-16471, Tabriz, Iran. 1 R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. INTRODUCTION MATERIALS AND METHODS Seminal plasma, which is a complex mixture secreted from the testes, epididymis and accessory sex glands, can affect sperm morphology, motility, acrosome reaction and fertility [29]. Despite its physiological significance, the molecular composition of the seminal plasma is complex and the various proteins or polypeptides present in seminal plasma are poorly understood. In recent years, several seminal plasma proteins have been identified, isolated and characterized. Evidence suggests that the protein composition of seminal plasma is different among species and that some seminal plasma proteins are associated with fertility in various species. Homologous proteins have also been identified in stallion [6], canine [10], goat [25], buffalo [1] and ram [18]. Also it is known that the seminal plasma contains substances that support the sperm cells. Sodium (Na+) and potassium (K+) cations in the seminal plasma establish the osmotic balance, while the essential trace elements are the components of many important enzymes [39], also calcium (Ca2+) is needed for stimulation of steriodogenesis in leyding cells of the testis [17 ]. Lactate dehydrogenase (LDH) plays an important role in the sperm metabolism, sperm capacitation and fertilization [12]. Alkaline phosphatase (ALP) is primary of testicular and epididymal origin and, therefore, suitable for differentiation of oligo - and azoospermia [35]. Likewise, ALP, AST is essential for metabolic processes which provide energy for survival, motility and fertility of spermatozoa. Previous studies are generally related to the comparisons of seminal plasma composition between males of different fertility or the isolation and characterisation of specific seminal proteins that could influence sperm capacitation and fertilisation. However, the correlation between specific seminal proteins and semen characteristics in crossbred rams with differing fertility has not hitherto been well studied. Therefore the objective of this study was to investigate enzyme activities (AST, ALP and LDH) and concentrations of Na+, K+ and Ca 2+. Moreover the present study was performed to investigate proteins in ram seminal plasma and the correlation between specific proteins and semen characteristics in cross breed ram. Animal and location This trial was performed at the sheep breeding Station, located in Tabriz, Iran. 16 crossbred fertile rams (4 Baluchi × Moghani; 4 Ghezel × Baluchi; 4 Ghezel × Merino; 4 Merino × Moghani), 2-3 years of age and with a live weight of 50-65 kg used in the study. The animals were maintained under natural photoperiod and during the trial, the rams were housed separately from the ewes. All rams received a daily diet of 666 g alfalfa hay, 66.5 g barley and 136.5 g of a commercial concentrate Semen collection and quality evaluation Forty eight Semen samples (three ejaculates of each animals) were collected, from the 16 fertile cross bred rams trained to serve an artificial vagina (AV) (temperature of 42-43°C). Prior to collection, the prepuce was wiped clean to prevent contamination of the semen. Semen samples were collected in the mornings, and transported to the laboratory (37°C), within 5-10 min, and placed in a water bath at 37°C. Semen volume was estimated directly in the calibrated semen collection tube, and sperm concentration determined with the aid of a Neubauer hemocytometer, after dilution (1:200) of the semen sample with a 2% eosin solution. To evaluate the sperm progressive motility, a sample of the diluted sperm was placed under a cover slip on a pre-warmed (37°C) slide and subjectively assessed using a phase contrast microscope ( × 400 magnification). The percentage live sperm was determined by evaluating 200 sperm/semen sample, (following eosin-nigrosin staining), under a light microscope ( × 400 magnification) [13]. At least 200 cells were counted in duplicates for each sample. Preparation of seminal plasma The seminal plasma was separated immediately after collection. Fresh semen was centrifuged at 1500 × g for 15 min at 5°C. The supernatants were transferred into 1.5 mL eppenddorf of tubes and recentrifuged at 14,000 × g for 10 min at 5°C to eliminate the remaining sperm. Seminal plasma was stored at -20°C until used. 2 R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. Seminal plasma compositions Statistical analysis Seminal plasma compositions were determined according to protocols presented in previous studies: Glucose [26], total protein [27], AST [32], ALP [23], LDH [2]. Na+, K+ and Ca2+ were also measured by Micro light1 based on ion-exchanger colorimetric method [37]. Data analysis was performed using SPSS software program (version 16.0 for Windows). All values were expressed as Mean ± standard error of mean (S.E.M.). Spearman’s correlation coefficient test was applied to examine the correlation between seminal plasma protein fractions with all the parameters of the semen. Differences were considered to be statistically significant at P < 0.05. SDS polyacrylamide denaturing gel electrophoresis (SDS-PAGE) The seminal plasma proteins were analysed by SDS–PAGE gel electrophoresis. Seminal plasma samples were suspended in loading buffer (4:1 v/v) containing 50 mM dithiotreitol, 20 mM Tris, 2.5% sodium dodecyl sulphate (SDS), 0.002% bromophenolblue and 5% glycerol, pH 6.8. Electrophoresis was performed in 15% separating and 5% stacking gels according to Laemmli [24] method. Each lane was loaded with 30 mL seminal plasma. Samples were concentrated at 70 V for 10 min; separation was performed at 120 V for 4 h. Gels were stained with Coomassie Brilliant Blue, G-250. RESULTS The results of the semen quality parameters of sixteen cross breed rams are summarized in Table 1, and depicted as mean ± S.E.M. Progressive motility(r = 0.993, P < 0.01) was correlated significantly with sperm viability. A significant negative correlations were observed among Sperm viability, progressive motility and sperm abnormality (r = -0.982 and r = -0.987, P < 0.01 respectively). Semen characteristics (semen volume, semen pH, sperm viability, sperm concentration and sperm motility,) were not significantly among cross breed rams. Mean values of some seminal plasma composition in four crossbreed rams are presented in Table 2. The highest seminal plasma concentration of Na+, K+ N and Ca2+ was recorded in Ghezel × Merinos rams. A Image acquisition Gel images were analysed to determine molecular weight and relative protein content using the Total Lab TL120 computer program (v2009, USA). Table 1. Semen characteristics in fresh ejaculates of cross breed rams with different fertility patterns. The similar superscript letters in the rows indicate non-significant differences. a significant negative correlation was observed between Na+ and K+ (r = -0.727 P < 0.05) but small (P < 0.05) correlation was recorded between Na+ and Ca2+ (r = 0.619 P < 0.05). The highest plasma concentration of ALP (Mean ± S.E.M) was recorded in Baluchi × Moghani ram and the lowest was recorded in Merino × Moghani ram (8 ± 1.25 and 3.33 ± 0.01 U/mL, respectively). The highest plasma concentration of LDH and AST (Mean ± S.E.M) was recorded in Ghezel × Baluchi ram (4.68 ± 0.19 U/mL, 19 ± 4 U/mL respectively) (Table 2). There was not correlation among, ALP and AST and semen characteristics. Significant correlations were found between LDH levels and sperm viability (r = 0.721, P < 0.05). The distribution of cross breed rams seminal plasma proteins is shown in Figure 1. Seventeen protein bands with different molecular weights ranging from 8.81 to 95.82 kDa were identified on the gel. (95.82, 85.35, 80.40, 74.24, 68.56, 62.89, 58.07, 50.52, 46, 34, 40.04, 33.92, 28.73, 19.04, 16.45, 13.48, 10.97, 8.81 kDa), twelve of these were presented in all samples 3 R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. Table 2. Least square means of concentrations of Na+, K+, Ca2+, Alcalin posphatase (ALP), Lactate dehydrogenase (LDH), Aspartate aminotranferase (AST) and Total protein (TP) in the seminal plasma of cross breed rams. Different superscript letters in the rows indicate significant differences P < 0.05. a,b Figure1. Molecular weight (kDa) of proteins of the seminal plasma (SP), the total protein fraction (TPF), as revealed by SDS–PAGE gel electrophoresis. Gels were stained with Coomassie Brilliant Blue. Numbers indicate the molecular weight of the standard in kDa. Lane 1( Merinos × Moghani) Lane 2(Merinos × Moghani) Lane 3 ( Baluchi × Moghani×) Lane 4( Baluchi × Moghani) Lane 5 (Ghezel ×Baluchi) Lane 6 ( Merinous × Moghani× ) Lane 7(Ghezel × Merinous) Lane 8 (Baluchi × Moghani) Lane 9 ( Meronos Moghani Lane 10( Marker). (Figure1). Five proteins in this study had quantitative difference between seminal plasma from cross breed rams. Spot 2 (85.35) was present in 7 samples, spot 7 (58.07) was shown in 7 samples, spot10 ( 40.04) was shown in 8 samples, spot 13 (19.04) was present in 8 samples and spot16 (10.97) just was shown in 2 samples. Those spots are shown in Figure 1. The 85.35 kDa, 40.04 kDa, 19.04 (Lane 1 and Lane 2) proteins were not detected in the semen samples obtained from Merinos × Moghani rams. The 58.07 kDa (Lane 5 and Lane 7) protein was not detected in the semen samples obtained from Ghezel × Baluchi and Ghezel × Merinous rams. No significant correlations were observed between protein bands and semen characteristics. plasma components and semen characteristics were investigated. Spermatological parameters were in the range of previous studies [16,20], even though minor differences were shown in some parameters. The mean ejaculate volume recorded for four cross breed rams, ranged between 1.0 and 1.4 mL, with the mean sperm concentration being between 3.3-4.7 × 109 sperm/ mL. The mean sperm motility and sperm viability in four cross breeds rams ranged between (67-86% and 65-75% respectively). This could be a reflection of various factors that may influence sperm production including genetics, environmental conditions and nutrition. This finding is in agreement with data on several sheep breeds in the temperate climates e.g. the Moghani rams [40], Ghezel and Mehraban breeds [39], and Persian Karakul rams in Iran [19]. In previous studies using SDS-PAGE, in a gradient (4-22%) of polyacrylamide gel, 20 bands DISCUSSION In the current study, the fertility-associated proteins and correlation between specific seminal 4 R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. were found in ovine seminal plasma and the most prominent bands were <70 kDa [3]. In the present study, seventeen protein fractions were detected in cross breed rams seminal plasma with molecular masses ranging from 8.81 to 95.82 kDa and the most prominent spots were those < 50 kDa. In the study by Jobim et al. [18], molecular weights ranged from 15 to 115 kDa and the most prominent spots were those < 30 kDa. The protein spots with a molecular weight from 15 to 20 kDa accounted for 41% of the relative intensity of the spots of the gel. Cardozo et al. [8] observed that when the molecular weights range between 12.5 and 83.9 kDa, the protein spots <21 kDa had the highest relative intensity using the gradient gel. In other study by Bergeron et al. [4], SDS-PAGE analysis of alcohol-precipitated ram seminal proteins indicated the presence of about 25 proteins with molecular masses from 14 to 120 kDa; a group of proteins with a molecular mass of 15-16 kDa and 22-24 kDa was more predominant. Also this is in agreement with those reported in the bull [28] and stallion [34]. So we came to the conclusion that most proteins in ram seminal plasma are below 50 kDa. The difference of molecular weight could be influenced by season, breeds, age and collecting and preparation methods of seminal plasma. Correlation between seminal plasma proteins and fertility of the male has been reported in some species of domestic animals such as bull [22] ram [18] goat [36] buffalo [1] stallion and boar [7]. The results showed that the relative content of five protein bands (85.35, 58.07, 40.04, 19.04 and 10.97 kDa) was significantly differences between seminal plasma cross breed rams. In a study by Killian et al. [22] showed that two of proteins 26 kDa and 55kDa associated with high fertility in bulls. However in our study protein band 58 kDa in Lane 5 and Lane 7 was not correlation with semen characteristics. Protein spot 10 (40 kDa) in lane 2 may correspond to clusterin. Clusterin is the major glycoprotein in ram retetestis fluid, with an apparent molecular mass of approximately 40 kDa on SDS-PAGE and an acidic isoelectric point (3.6). The highest tissue concentrations of clusterin are present in the testis and epididymis; Sertoli cells may be the testicular source of clusterin[5]. It is involved in sperm maturation [33], and possesses heparin-binding sites [18] suggested that this protein, present in bull seminal plasma, is related to high semen freezability. Protein spot 13 (19 kDa, lane 2) may be TIMP-2 (tissue inhibitor of metalloproteinase-2), a potent inhibitor of the matrix-metalloproteinases, present in the caput epididymal fluid in the ram, boar and stallion [30]. The TIMPs are specific inhibitors of matrix metalloproteinases (MMPs), which are generally present at the same time as the matrix proteases. These finding suggest that some proteins may modulate sperm function by providing energy and protection for spermatozoa as a complementary substance. Yue et al. [38] reported that protein band 72.45 kDa was correlated with sperm viability. Although the other seven protein bands of all seminal plasma samples showed no fertility associated change in their relative content. LP is a dephosphorylating enzyme that is active in many tissues including bone, liver, kidney, intestine, lung and placenta. The majority of ALP in rams originates from the seminal vesicles and, to a lesser extent, from the testes and epididymides. Dogan et al. [11] showed a significant positive correlation with concentration, AST and LDH, but not with volume in bull seminal plasma. While, we couldn’t found the correlation between ALP and semen characteristics. In our study significant correlations were N found between LDH levels and sperm viability. This study is in agreement with other results. Zamiri and Khodaei [39] showed that correlation coefficient of the LDH level with the percentage live sperm (r = −0.51) was however smaller than the value reported for fat-tailed Iranian rams (r = −0.75). In this study every increase in the percentage of live and normal sperm corresponded to an increase in LDH activity in the seminal fluid. It has been stated that LDH plays an important metabolic role in sperm capacitation and fertilization [12]. Furthermore, LDH is an intracellular enzyme and increased levels in the seminal fluid may be an indication of the integrity of the sperm plasma membrane. In the present study we couldn’t found a correlation between AST and semen characteristics. This information is consistent with finding Pesch et al. [31] who reported that negative correlation was between AST enzyme and sperm volume. Thus, increasing the percentage of abnormal spermatozoa in ejaculate causes high concentration of transaminase enzyme in the extra cellular fluid due to sperm membrane damage and ease of leakage of enzymes from 5 R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. spermatozoa [15]. However, Corteel [9] reported that the transaminase activities (AST-ALT) in semen are good indicators of semen quality because they measure sperm membrane stability. Our result showed that seminal Na+, K+ concentrations were higher than the range recorded for other fat-tailed sheep breeds [40]. In the present study, the high levels of Na+ and K+ ions were associated with low percentages of motile sperm, and such semen was considered to be of lower quality. This result is contrast to previous finding. Zamiri and Khodaei [39] showed that low levels of Na+ and K+ ions were associated with high percentage of motile sperm. This study suggest that the cations Na+ and K+ generally establish the osmotic balance, and seminal plasma osmolality ultimately plays an important role in the activation sperm cell also potassium ions (K+) are also intracellular cations, and the concentrations in the seminal fluid may be an indication of sperm plasma membrane integrity. Our result showed that high levels of Ca2+ were associated with lower percentage of motile sperm. This is agreement with the report of Kaya et al. [21] who showed that increasing ejaculation frequency due to reduction Ca2+seminal plasma and decrease in sperm motility. Garcia and Graham [14] showed that reverse proportional correlation existed between Ca2+ content and the motility of seminal cells. In conclusion our results indicate that relative content of seminal plasma could not be an essential index to evaluate ram semen quality. While some enzyme and mineral composition could be an essential index to evaluate cross breed ram semen quality. However, further studies and more experimental data are needed to construct reliable mathematical models which would make it possible to develop a simple method for the prediction of ram fertility. SOURCE AND MANUFACTURER 1 PL1000B VTT, Technical Research Centre of Finland Vuorimiehentie, Finland. Declaration of interest. The author report no conflicts of interest, and alone is responsible for the content and writing of the paper. REFERENCES 1 Asadpour R., Alavi-Shoushtari S.M., Asri Rezaii S. & Ansari M.H.K.H. 2007. SDS-polyacrylamide gel electrophoresis of buffalo bulls seminal plasma proteins and their relation with semen freezability. Animal Reproduction Science. 102(3-4): 308-313. 2 Babson A.L. & Babson S.R. 1973. Kinetic Colorimetric Measurement of Serum Lactate Dehydrogenase Activity. Clinical Chemistry. 19(7): 766-769. 3 Barrios B., Pérez-Pé R., Gallego M., Tato A., Osada J. & Muiño-Blanco T. 2000. Seminal plasma proteins revert the cold-shock damage on ram sperm membrane. Biology of Reproduction. 63(5): 1531-1537. 4 Bergeron A., Villemure M., Lazure C. & Manjunath P. 2005. Isolation and characterization of the major proteins of ram seminal plasma. Molecular Reproduction and Development. 71(4): 461-470. 5 Blaschukz O., Burdzy K. & Fritz I.B. 1983. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. Journal of Biological Chemistry. 25(12): 7714-7720. 6 Brandon C.I., Heusner G.L., Caudle A.B. & Fayrer-Hosken R.A. 1999. Two-dimensional polyacrylamide gel electrophoresis of equine seminal plasma proteins and their correlation with fertility. Theriogenology. 52(5): 863-873. 7 Calvete J.J., Campanero-Rhodes M.A., Raida M. & Sanz L. 1999. Characterisation of the conformational and quaternary structure-dependent heparin-binding region of bovine seminal plasma protein PDC-109. FEBS Lett. 444(2): 260-264. 8 Cardozo J.A., Fernández-Juan M., Forcada F., Abecia A., Muiño-Blanco T. & Cebrián-Pérez J.A. 2006. Monthly variations in ovine seminal plasma proteins analyzed by two-dimensional polyacrilamide gel electrophoresis. Theriogenology. 66(4): 841-850. 9 Corteel J.M. 1980. Effets du plasma séminal sur la survie et la fertilité des spermatozoïdes conservés in vitro. Reproduction Nutrition Développement. 20(4): 1111-1123. 10 de Souza F.F., Barreto C.S. & Lopes M.D. 2007. Characteristics of seminal plasma proteins and their correlation with canine semen analysis. Theriogenology. 68(1): 100-106. 11 Dogan I., Polat U. & Nur Z. 2009. Correlations between seminal plasma enzyme activities and semen parameters in seminal fluid of Arabian horses. Iranian Journal of Veterinary Research. 10(2): 119-124. 6 R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. 12 Duan C. & Goldberg E. 2003. Inhibition of lactate dehydrogenase C4 (LDHC4) blocks capacitation of mouse sperm in vitro. Cytogenetic and Genome Research. 103(3-4): 352-359. 13 Evans G. & Maxwell W.M.C. 1987. Salamon’s Artificial Insemination of Sheep and Goats. Sydney: University Press, 200p. 14 Garcia M.A. & Graham E.F. 1989. Development of a buffer system for dialysis of bovine spermatozoa before freezing. III. Effect of different inorganic and organic salts on fresh and frozen-thawed semen. Theriogenology. 31(5): 1039-1048. 15 Gundogan M. & Serteser M. 2005. Some reproductive parameters and biochemical properties in Akkaraman and Awassi rams. Turkish Journal Veterinary Animal Science. 29(1): 595-599. 16 Gundogan M. 2007. Seasonal variation in serum testosterone, T3 and andrological parameters of two Turkish sheep breeds. Small Ruminant Research. 67(2-3): 312-316. 17 Henricks D.M. 1991. Biochemistry and physiology of the gonadal hormones. In: Cupps P.T. (Ed). Reproduction in Domestic animals. 4th edn. San Diego: Academic Press, 670p. 18 Jobim M.I., Oberst E.R., Salbego C.G., Wald V.B., Horn A.P. & Mattos R.C. 2005. BSP A1/A2-like proteins in ram seminal plasma. Theriogenology. 63(7): 2053-2062. 19 Kafi M., Safdarian M. & Hashemi M. 2004. Seasonal variation in semen characteristics, scrotal circumference and libido of Persian Karakul rams: technical note. Small Ruminant Research. 53(1-2): 133-139. 20 Karagiannidis A., Varsakeli S., Alexopoulos C. & Amarantidis I. 2000. Seasonal variation in semen characteristics of Chios and Friesian rams in Greece. Small Ruminant Research. 37(1-2): 125-130. 21 Kaya A., Askoy M. & Tekeli T. 2002. Influence of ejaculation frequency on sperm characteristics, ionic composition and enzymatic activity of seminal plasma in rams. Small Ruminant Research. 44(2): 153-158. 22 Killian G.J., Chapman D.A. & Rogowski L.A. 1993. Fertility associated proteins in Holstein bull seminal plasma. Biology of Reproduction. 49(6): 1202-1207. 23 Kind P.R.N. & King E.J. 1954. Estimation of plasma phosphatase by determination of hydrolysed phenol with antipyrine. Journal of Clinical Pathology. 7(4): 322-326. 24 Laemmli U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 15(1): N 680-685. 25. La Falcia V.S.N. Tortorella H., Rodrigues J.L. & Brandelli A. 2002. Seasonal variation of goat seminal plasma proteins. Theriogenology. 57(3):1035-1048. 26 Lott J.A. & Turner K. 1975. Evaluation of Trinder’s Glucose Oxidase Method for Measuring Glucose in Serum and Urine. Clinical Chemistry. 21(12): 1754-1760. 27 Lowry O.H., Rosebrough N.J., Farr A.L. & Randall R.J. 1951. Protein measurement with folin phenol reagent. Journal of Biological Chemistry. 193(1): 265-275. 28 Manjunath P. & Sairam M.R. 1987. Purification and biochemical characterization of three major acidic proteins (BSP-A1, BSP-A2 and BSP-A3) from bovine seminal plasma. Biochemical Journal. 241(2): 685-692. 29 Mann T. & Lutwak-Mann C. 1981. Male Reproductive Function and Semen. Berlin: Springer-Verlag, 495p. 30 Métayer S., Dacheux F., Dacheux J.L. & Gatti J.L. 2002. Comparison, Characterization, and Identification of Proteases and Protease Inhibitors in Epididymal Fluids of Domestic Mammals. Matrix Metalloproteinases Are Major Fluid Gelatinases. Biology of Reproduction. 66(5): 1219-1229. 31 Pesch S., Bergmann M. & Bostedt H. 2006. Determination of some enzymes and macroand microelements in stallion seminal plasma and their correlations to semen quality. Theriogenology. 66(2): 307-313. 32 Rietman S. & Frankel S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 28(1): 56-63. 33 Sylvester C., Morales R., Oko R. & Griswold M.D. 1991. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biology of Reproduction. 45(1):195-207. 34 Topfer-Petersen E., Ekhlasi-Hundrieser M., Tsolova, M., Leeb T., Kirchhoff C. & Muller P. 2005. Structure and function of secretory proteins of the male genital tract. Andrologia. 37(6): 202-204. 35 Turner R.M. & McDonnell S.M. 2003. Alkaline phosphatase in stallion semen: haracterization and clinical applications. Theriogenology. 60(1): 1-10. 36 Villemure M., Lazure C. & Manjunath P. 2003. Isolation and characterization of gelatin-binding proteins from goat seminal plasma. Reproductive Biology and Endocrinology. 39 (1): 39-50. 7 R. Asadpour. 2012. Relationship between Mineral Composition of Seminal Plasma and Semen Quality in Various Ram Breeds. Acta Scientiae Veterinariae. 40(2): 1027. 37 Yoshimura K., Waki H. & Ohashi S. 1976. Ion-exchanger colorimetry-I Micro determination of chromium, iron, copper and cobalt in water. Talanta. 23(6): 449-454. 38 Yue W., Shi L., Bai Z., Ren Y. & Zhao Y. 2009. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis of ram seminal plasma proteins and their correlation with semen characteristics. Animal Reproduction Science. 116(3-4): 386-391. 39 Zamiri M.J. & Khodaei H.R. 2005. Seasonal thyroidal activity and reproductive characteristics of Iranian fat-tailed rams. Animal Reproduction Science. 88(3-4): 245-255. 40 Zamiri M.J., Khalili B., Jafaroghli M. & Farshad A. 2010. Seasonal variation in seminal parameters, testicular size, and plasma testosterone concentration in Iranian Moghani rams. Small Ruminant Research. 94(1-3): 132-136. Pub. 1027 www.ufrgs.br/actavet 8