Ionic Compounds: Formulas, Bonds, & Properties Worksheet
advertisement
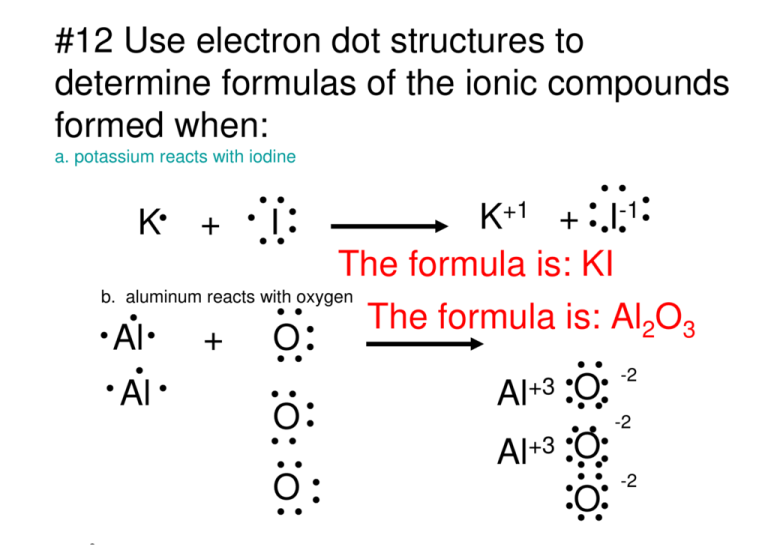
#12 Use electron dot structures to determine formulas of the ionic compounds formed when: a. potassium reacts with iodine +1 + I-1 K K + I The formula is: KI b. aluminum reacts with oxygen The formula is: Al2O3 Al + O -2 +3 Al Al O -2 O Al+3 O -2 O O #13 What is the formula of the ionic compound composed of calcium and chloride anions? Cl Ca Cl Ca+2 Cl-1 You need 1 calcium and 2 chlorines +2 Ca and -1 Cl Cl-1 Ca+2 Ca1Cl2 The final formula is CaCl 2 #14 How can you describe the electrical charge of an ionic compound? The total positive charge exactly balances the total -1 I negative charge making the compound Sr electrically neutral. I-1 +2 (+2) + (-1) + (-1) = 0 #15 What properties characterize ionic compounds? 1.Ionic Compounds Often Have High Melting and Boiling Points The attraction between cations and anions is often very strong and hard to break. Cation Anion 2. Ionic Compounds Form Crystals 3. Ionic Compounds are hard and brittle when hit. Metal: “sea of electrons” Attract Repel 4. Ionic Compounds Conduct Electricity when dissolved in Water or Melted. #16 define an ionic bond. Ionic Bonding animation Another NaCl animation NaCl animation An Ionic bond is when oppositely charged ions form electrostatic bonds: Example: NaCl The Na+1 and the Cl-1 being oppositely charged, attract each other. #17 How can you represent the composition of an ionic compound? You write its chemical formula: example Rb2S NaCl Fe2(SO4)2 #18 Write the correct chemical formula for the compounds formed from each pair of ions. a. K+1 and S-2 K2S b. Ca+2 and O-2 Ca2O2 CaO c. Na+1 and O-2 Na2O d. Al+3 and N-3 Al3N3 AlN #19 Write the formulas for each compound: a. barium chloride BaCl2 Ba+2 Cl-1 b. magnesium oxide MgO Mg+2 O-2 c. lithium oxide Li+1 Li2O O-2 d. calcium fluoride -1 +2 F Ca CaF2 # 20 Which pairs of elements are likely to form ionic compounds? a. Cl, Br NO, they are both nonmetals! b. Li, Cl Yes, they will form LiCl c. K, He d. I, Na NO, Helium doesn’t combine with anything! Yes, they form NaI #21 Describe the arrangement of sodium ions and chloride ions in a crystal of sodium chloride. Empirical Formula: The smallest whole number ratio of atoms in a compound--- NaCl #22 Why do ionic compounds conduct electricity when they are melted or dissolved in water? Conductivity salt dissolving The ions are free to move around and thus they can migrate to the opposite poles and exchange electrons. http://www.chem.iastate.edu/group/Greenbowe/sections/projectfolder/flashfiles/thermoch animations of coordination number http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/flash.mhtml Metallic Bond: "The SEA OF ELECTRONS" Bonding by analogy of Dog bones




