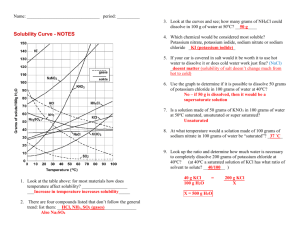
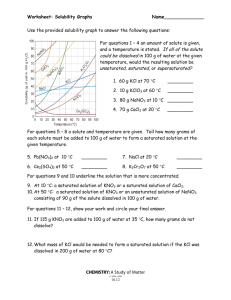
2. G, how 1. 5 g 3. 15 g 3.
advertisement

Solutions Review Questions Name: ____________________________________ Thursday, March 06, 2008 1. Compared to pure water, an aqueous solution of calcium chloride has a 1. higher boiling point and higher freezing point 2. higher boiling point and lower freezing point 3. lower boiling point and higher freezing point 4. lower boiling point and lower freezing point 2. An unsaturated aqueous solution of NH 3 is at 90°C in 100 grams of water. According to Reference Table G, how many grams of NH3 could this unsaturated solution contain? 1. 5 g 3. 15 g 2. 10. g 4. 20. g 3. Solubility data for four different salts in water at 60°C are shown in the table below. Salt Solubility in Water at 60°C A 10 grams / 50 grams H2O B 20 grams / 60 grams H2O C 30 grams / 120 grams H2O D 40 grams / 80 grams H2O Which salt is most soluble at 60°C? 1. A 2. B 3. C 4. D Solutions Review Questions 4. Which compound is insoluble in water? 1. BaSO4 3. KClO3 2. CaCrO4 4. Na2S 5. Which solution containing 1 mole of solute dissolved in 1000 grams of water has the lowest freezing point? 1. KOH(aq) 2. C2H12O6(aq) 3. C2H5OH(aq) 4. C12H22O11(aq) 6. According to Reference Table G, which of these substances is most soluble at 60°C? 1. NaCl 2. KCl 3. KClO3 4. NH4Cl 7. How many moles of solute are contained in 200 milliliters of a 1 M solution? 1. 1 2. 0.2 3. 0.8 4. 200 8. Which sample of matter is classified as a solution? 1. H2O(s) 2. H2O(l) 3. CO2(g) 4. CO2(aq) Solutions Review Questions 9. According to Reference Table G, which compound's solubility decreases most rapidly when the temperature increases from 50°C to 70° C? 1. NH3 2. HCl 3. SO2 4. KNO3 10. Base your answer to the question on the information below. A student is instructed to make 0.250 liter of a 0.200 M aqueous solution of Ca(NO3)2. Figure 1 What is a correct numerical setup for calculating the total number of moles of Ca(NO 3)2 needed to make 0.250 liter of the 0.200 M calcium nitrate solution? 1. 2. 3. 4. 11. According to Reference Table G, which of the following substances is least soluble in 100 grams of H2O(l) at 50°C? 1. KCl 2. NaCl 3. NH4Cl 4. HCl Solutions Review Questions 12. A student prepares four aqueous solutions, each with a different solute. The mass of each dissolved solute is shown in the table below. Which solution is saturated? 1. 1 3. 3 2. 2 4. 4 13. Based on Reference Table F, which of these saturated solutions has the lowest concentration of dissolved ions? 1. NaCl(aq) 2. MgCl2(aq) 3. NiCl2(aq) 4. AgCl(aq) 14. At STP, which of these substances is most soluble in H2O? 1. CCl4 2. CO2 3. HCl 4. N2 Solutions Review Questions 15. According to Reference Table G, a temperature change from 60°C to 90°C has the least effect on the solubility of 1. SO2 2. NH3 3. KCl 4. KClO3 16. A safe level of fluoride ions is added to many public drinking water supplies. Fluoride ions have been found to help prevent tooth decay. Another common source of fluoride ions is toothpaste. One of the fluoride compounds used in toothpaste is tin(II) fluoride. A town located downstream from a chemical plant was concerned about fluoride ions from the plant leaking into its drinking water. According to the Environmental Protection Agency, the fluoride ion concentration in drinking water cannot exceed 4 ppm. The town hired a chemist to analyze its water. The chemist determined that a 175-gram sample of the town's water contains 0.000 250 gram of fluoride ions. How many parts per million of fluoride ions are present in the analyzed sample? ppm 17. Based on Reference Table G, which salt solution could contain 42 grams of solute per 100 grams of water at 40°C? 1. a saturated solution of KClO3 2. a saturated solution of KCl 3. an unsaturated solution of NaCl 4. an unsaturated solution of NH4Cl 18. How many liters of a 0.5 M sodium hydroxide solution would contain 2 moles of solute? 1. 1L 2. 2L 3. 3L 4. 4L Solutions Review Questions 19. Figure 2 A student obtained the data in the table in a chemistry laboratory. Based on Reference Table G, which of the trials seems to be in error? 1. 1 2. 2 3. 3 4. 4 20. At room temperature, the solubility of which solute in water would be most affected by a change in pressure? 1. methanol 2. sugar 3. carbon dioxide 4. sodium nitrate 21. What is the total number of grams of NaOH (formula mass = 40.) needed to make 1.0 liter of a 0.20 M solution? 1. 20. g 2. 2.0 g 3. 80. g 4. 8.0 g 22. Based on Reference Table F, which salt is the strongest electrolyte? 1. CaCO3 2. Na2SO4 3. AgCl 4. Zn3(PO4)2 Solutions Review Questions 23. An aqueous solution contains 300. parts per million of KOH. Determine the number of grams of KOH present in 1000. grams of this solution. Answer: g 24. According to Reference Table F, which of these compounds is the least soluble in water? 1. K2CO3 2. KC2H3O2 3. Ca3(PO4)2 4. Ca(NO3)2 25. A saturated solution of NaNO 3 is prepared at 60°C using 100. grams of water. As this solution is cooled to 10°C, NaNO3 precipitates (settles) out of the solution. The resulting solution is saturated. Approximately how many grams of NaNO3 settled out of the original solution? 1. 46 g 3. 85 g 2. 61 g 4. 126 g 26. A 0.100-molal aqueous solution of which compound has the lowest freezing point? 1. C6H12O6 2. CH3OH 3. C12H22O11 4. NaOH 27. A 3.0 M HCl(aq) solution contains a total of 1. 3.0 grams of HCl per liter of water 3. 3.0 moles of HCl per liter of solution 2. 3.0 grams of HCl per mole of solution 4. 3.0 moles of HCl per mole of water Solutions Review Questions 28. According to Reference Table G, which solution at equilibrium contains 50 grams of solute per 100 grams of H2O at 75°C? 1. an unsaturated solution of KCl 2. an unsaturated solution of KClO3 3. a saturated solution of KCl 4. a saturated solution of KClO3 29. In aqueous solution, a chloride ion is attracted to which end of the water molecule? 1. the hydrogen end, which is the positive pole 2. the hydrogen end, which is the negative pole 3. the oxygen end, which is the positive pole 4. the oxygen end, which is the negative pole 30. What is the total number of grams of NaI(s ) needed to make 1.0 liter of a 0.010 M solution? 1. 0.015 2. 0.15 3. 1.5 4. 15 31. What is the molarity of a solution containing 20 grams of NaOH in 500 milliliters of solution? 1. 1 M 2. 2 M 3. 0.04 M 4. 0.5 M 32. What occurs when NaCl(s ) is added to water? 1. The boiling point of the solution increases, and the freezing point of the solution decreases. 2. The boiling point of the solution increases, and the freezing point of the solution increases. 3. The boiling point of the solution decreases, and the freezing point of the solution decreases. 4. The boiling point of the solution decreases, and the freezing point of the solution increases. Solutions Review Questions 33. What is the total number of moles of solute in 250 milliliters of a 1.0 M solution of NaCl? 1. 1.0 mole 2. 0.25 mole 3. 0.50 mole 4. 42 moles 34. A student adds solid KCl to water in a flask. The flask is sealed with a stopper and thoroughly shaken until no more solid KCl dissolves. Some solid KCl is still visible in the flask. The solution in the flask is 1. saturated and is at equilibrium with the solid KCl 3. unsaturated and is at equilibrium with the solid KCl 2. saturated and is not at equilibrium with the solid 4. unsaturated and is not at equilibrium with the solid KCl KCl 35. An aqueous solution has 0.0070 gram of oxygen dissolved in 1000. grams of water. Calculate the dissolved oxygen concentration of this solution in parts per million. Answer: ppm 36. When 20. milliliters of 1.0 M HCL is diluted to a total volume of 60. milliliters, the concentration of the resulting solution is 1. 1.0 M 2. 0.50 M 3. 0.33 M 4. 0.25 M 37. What is the molarity of a solution that contains 30. grams of NaOH in 500. milliliters of solution? 1. 0.75 M 2. 1.3 M 3. 1.5 M 4. 2.6 M Solutions Review Questions 38. The solubility of KClO3(s) in water increases as the 1. temperature of the solution increases 2. temperature of the solution decreases 3. pressure on the solution increases 4. pressure on the solution decreases 39. Hexane (C6H14) and water do not form a solution. Which statement explains this phenomenon? 1. Hexane is polar and water is nonpolar. 2. Hexane is ionic and water is polar. 3. Hexane is nonpolar and water is polar. 4. Hexane is nonpolar and water is ionic. 40. Which concentration of a solution of CH3OH in water has the lowest freezing point? 1. 0.1 M 2. 0.01 M 3. 0.001 M 4. 0.0001 M 41. The water solution of which of the following substances is the best conductor of electricity? 1. KCl 2. C6H12O6 3. CO2 4. CO 42. Which statement is true for a saturated solution? 1. It must be a concentrated solution. 2. It must be a diluted solution. 3. Neither dissolving nor crystallizing is occurring. 4. The rate of dissolving equals the rate of crystallizing. Solutions Review Questions 43. As the pressure on a gas confined above a liquid increases, the solubility of the gas in the liquid 1. decreases 2. increases 3. remains the same 44. What is the total number of moles of H2SO4 needed to prepare 5.0 liters of a 2.0 M solution of H2SO4? 1. 2.5 2. 5.0 3. 10. 4. 20. 45. Which 0.1 M solution contains an electrolyte? 1. C6H12O6(aq) 2. CH3 COOH(aq) 3. CH3 OH(aq) 4. CH3OCH3 (aq) 46. Compared to a 2.0 M aqueous solution of NaCl at 1 atmosphere, a 3.0 M aqueous solution of NaCl at 1 atmosphere has a 1. lower boiling point and a higher freezing point 3. higher boiling point and a higher freezing point 2. lower boiling point and a lower freezing point 4. higher boiling point and a lower freezing point 47. An example of a physical property of an element is the element's ability to 1. react with an acid 2. react with oxygen 3. form a compound with chlorine 4. form an aqueous solution Solutions Review Questions 48. What is the molarity of a solution of NaOH if 2 liters of the solution contains 4 moles of NaOH? 1. 0.5 M 2. 2 M 3. 8 M 4. 80 M 49. If 0.025 gram of Pb(NO3)2 is dissolved in 100. grams of H2O, what is the concentration of the resulting solution, in parts per million? 1. 2.5 ×10-4 ppm 3. 250 ppm 2. 2.5 ppm 4. 4.0 ×103 ppm 50. Under which conditions are gases most soluble in water? 1. high pressure and high temperature 2. high pressure and low temperature 3. low pressure and high temperature 4. low pressure and low temperature Solutions Review Questions Answer Key for Solutions Review Questions 1. 2 2. 1 3. 4 4. 1 5. 1 6. 4 7. 2 8. 4 9. 1 10. 3 11. 2 12. 2 13. 4 14. 3 15. 1 16. 1.43 17. 4 18. 4 19. 4 20. 3 21. 4 22. 2 23. 0.300 g 24. 3 25. 1 26. 4 27. 3 28. 3 29. 1 30. 3 31. 1 32. 1 33. 2 34. 1 35. 7.0 36. 3 37. 3 38. 1 39. 3 40. 1 41. 1 42. 4 43. 2 44. 3 45. 2 46. 4 Solutions Review Questions 47. 4 48. 2 49. 3 50. 2