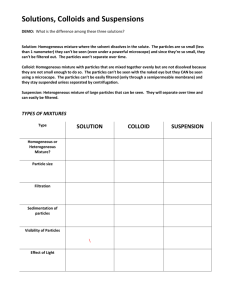
Conclusion : Foul Water The purpose of the lab is to purify a sample
advertisement

Conclusion : Foul Water The purpose of the lab is to purify a sample of foul water so that it is usable for hand washing. First, a dropper was used to separate the oil layer which was found floating on top of the water due to its lower density. Next, sand‐gravel filtration was used to remove large particles. The filtrate, the mixture obtained after passing through the sand and gravel, was added to charcoal which adsorbed particles onto its surface to remove odor and color.The charcoal‐foul water mixture was filtered to remove the charcoal and other fine particles. The percent of foul water recovered was only 66%. Some of the water could have been accidentally removed by the dropper when extracting the oil. The purified water was more clear than the original sample. It was also odor free and contained no solid particles. Electrical conductivity was not tested. If salt particles were present, they were dissolved in the mixture and could not be removed by any process in this lab. Distillation, however, if available could have removed the salt.