Chapter 24
advertisement

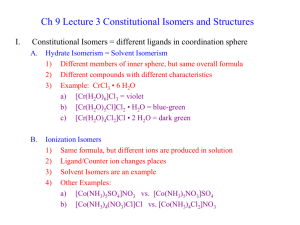
Transition Metals & Coordination Compounds Gemstones The colors of rubies and emeralds are both due to the presence of Cr+3 ions – the difference lies in the crystal hosting the ion In rubies, some Al+3 ions in the Al2O3 are replaced by Cr+3 ions. In emeralds, some Al+3 ions in the Be3Al2(SiO3)6 are replaced by Cr+3 ions. Electron Configuration For 1st & 2nd transition series = ns2 (n−1)dx Fe = [Ar]4s23d6; Zr = [Kr]5s24d2 For 3rd transition series = ns2 (n−2)f14 (n−1)dx Re = [Xe] 6s2 4f14 5d5 Some individuals deviate from the general pattern by “promoting” one or more s electrons into the underlying d to complete the subshell Form ions by losing the ns electrons first, then the (n – 1)d Lewis Acids & Bases Section 15.11 G.N. Lewis – noticed that acid-base chemistry always involves an electron pair. BH3 + NH3 H3B:NH3 Acid = electron pair acceptor Base = electron pair donor Greatly expands what we view as an “acid” LEP #1 Complexes An ion like [Ag(NH3)2]+1, are called complex ions as well as coordination compounds. The molecules or ions that bond to the metal are known as ligands. The coordination sphere is the metal and the total number of ligands bonded to it. The complex is a neutral charge salt, which may contain additional cations or anions not bonded to the metal. Complexes [Cu(NH3)4] SO4 The complex ion charge is _____. The charge of Copper is _____. Coordination number is the number of lone pairs donated to the metal. Coordination numbers of 2, 4, and 6 are most common. Complexes Molecular Geometry Chelates Ligands are sometimes referred to as chelates (Greek = claw). Most are monodentate (one “toothed”) like NH3, Cl-, CN-, etc. A few are bidentate (two “toothed”) like ethylenediamine and the oxalate ion. A few are polydentate like EDTA. Chelates The formation of complexes favors the products as seen in Chapter 17. Ni+2(aq) + 6 NH3(aq) Ni(NH3)6+2 ; Kf = 4 E8 Ni+2(aq) + 3 en(aq) Ni(en)3+2(aq) ; Kf = 2 E18 The larger K for the bidentate ligand is known as the chelating effect. Uses of EDTA and the EDTA challenge. Metals in Living Systems Nine metals important to life – V, Cr, Mn, Fe, Co, Ni, Cu, Zn, and Mo – owe their roles to their ability to form complexes with ligands. The role of iron in hemeglobin is a perfect example. In hemeglobin, the iron is bonded to four N atoms in a molecule called porphoryn. The fifth site is bonded to the protein (globin). This leaves one position empty in the octahedral geometry. Metals in Living Systems Porphine molecule Nomenclature Complexes are named using a systematic method. Rules: 1. 2. 3. 4. Cation named first, then anion Name of the complex is always one word, name of ligands come first and in alphabetical order Name of ligands include prefixes if more than one Anionic ligands get an –o suffix Name of metal also includes oxidation number in ( ). If complex is an anion, metal name ends in –ate. Ex) Vandium = Vanadate, Ferrum = Ferrate Note: Some metals use old Latin names! Nomenclature LEP #2, #3 Isomers Isomers are compounds with the same formula but either atoms are in a different order (structural) or atoms are in a different spatial arrangement (stereoisomers). Structural Isomers A linkage isomer occurs when a ligand can bond through a different atom. NO2- can bond through the N (NO2-) or the O (ONO-). Another one is SCN-. Structural Isomers A coordination sphere isomer occurs when the ligands bonded to the metal are exchanged for ones outside of the coordination sphere. For example, the formula CrCl36H2O has several forms. [Cr(H2O)6] Cl3 is purple [Cr(H2O)5Cl] Cl2H2O is green Stereoisomers A geometric isomer occurs when the spatial orientation of a complex can be changed. These are referred to as cis-trans isomers. Example is the square planar geometry of PtCl2(NH3)2. Stereoisomers Can also produce cis-trans for octahedral complexes if general formula is: MX4Y2. Example is Co(NH3)4Cl2+. Stereoisomers A second type of geometric isomerism can occur if the general formula is MX3Y3 called fac-mer (short for facial and meridian). An example is Co(NH3)3Cl3. Stereoisomers An optical isomer occurs when the mirror image of the complex is non-superimposable. The pair of isomers are called enantiomers. Stereoisomers In complexes, the only way to get optical isomerism is with a 6-coordinate system and two or three bidentate ligands. Most of the chemical and physical properties of any enantiomer pair are identical. However, towards other optically active molecules only one might react. Stereoisomers If the two mirror image complex ions can be separated, then they can be tested with plane polarized light. Color Some ions are highly colored. Cu+2 = blue Ni+2 = green Co+2 = pink Some ions are not colored. Zn+2 Ba+2 Al+3 Color Color depends on two factors: 1. 2. _______________ _______________ Compounds must absorb some visible light to have a color. Color A compound’s color can be due to: either it absorbs all wavelengths but that color OR, it absorbs one color exclusively For the second choice, the color is then the complimentary color. Spectrum for Ti(H2O)6+3 Color Wheel The color wheel shows the complimentary colors. Those that are opposite are complimentary. Spectrum of +2 Ni Electron Configurations In period 4, the d orbitals start with Sc. Sc: [Ar] 4s2 3d1 Orbital diagram – shows how each of the d orbitals are filled. Example) Fe: [Ar] 4s2 3d6 Will see many metal ions, so that means you have to remove some of the electrons. Co+3 Magnetism Unpaired electrons = paramagnetic Paired electrons = diamagnetic Zn(Cl4)-2 = diamagnetic CoF6-3 = paramagnetic Co(CN)6-3 = diamagnetic ??? Crystal Field Theory (CFT) As the ligand donates its electron pair to form the bond, it interacts with the metal’s d orbitals. Not all the d orbitals are affected in the same way. This splits the d orbitals into different levels. d orbitals d orbitals CFT CFT CFT High and Low Spin Normally, electrons fill the d orbitals one at a time WITH parallel spins. Octahedral complexes Small D = fill each level first before pairing Large D = fill the lower level completely before moving to upper level only matters for d4 to d7 configurations High and Low Spin [CoF6]-3 [Co(CN)6]-3 They are different! Spectrochemical Series Ranks the ligands from weak to strong field. Cl- < F- < H2O < NH3 < en < NO2- < CN increasing D Tetrahedral and Square Planar D is always small for tetrahedral complexes so these are always high spin. D is always large for square planar complexes so these are always low spin. Tetrahedral and Square Planar Ni+2 can be either d8 [NiCl4]-2 [Ni(CN4)]-2 Measuring Delta D = hc / l Remember, though, if a compound is red, then it absorbs green. Use wavelength in green part of the spectrum!