Nomenclature of Ionic Compounds (ANSWER KEY)
advertisement
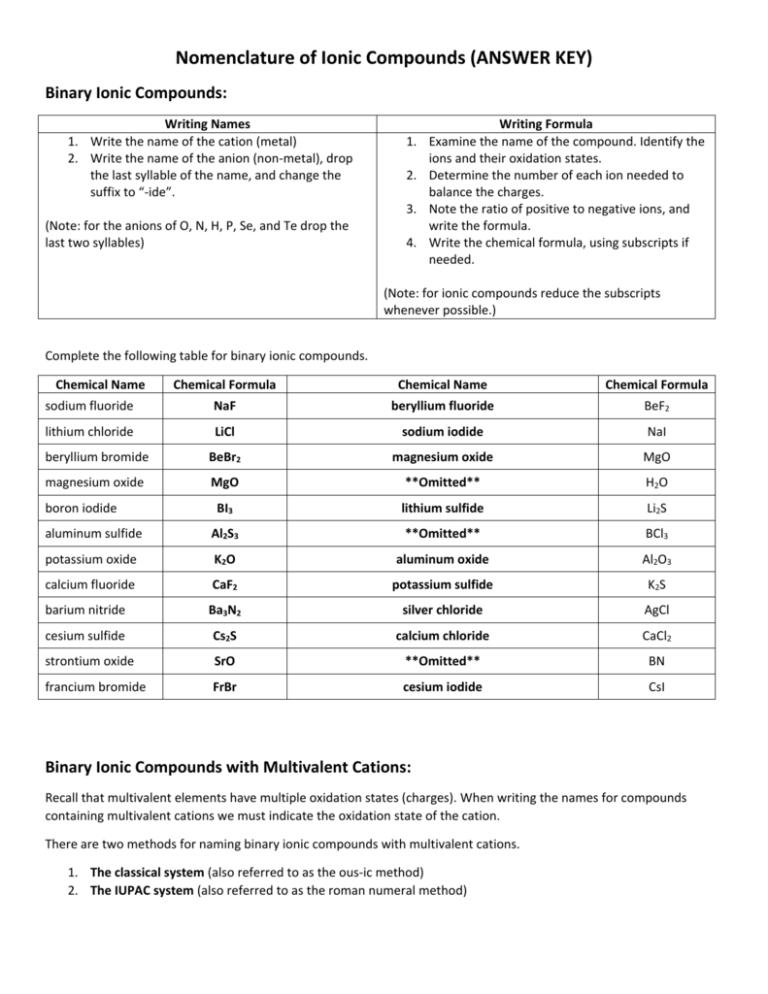
Nomenclature of Ionic Compounds (ANSWER KEY) Binary Ionic Compounds: Writing Names 1. Write the name of the cation (metal) 2. Write the name of the anion (non-metal), drop the last syllable of the name, and change the suffix to “-ide”. 1. 2. 3. (Note: for the anions of O, N, H, P, Se, and Te drop the last two syllables) 4. Writing Formula Examine the name of the compound. Identify the ions and their oxidation states. Determine the number of each ion needed to balance the charges. Note the ratio of positive to negative ions, and write the formula. Write the chemical formula, using subscripts if needed. (Note: for ionic compounds reduce the subscripts whenever possible.) Complete the following table for binary ionic compounds. Chemical Name Chemical Formula Chemical Name Chemical Formula sodium fluoride NaF beryllium fluoride BeF2 lithium chloride LiCl sodium iodide NaI beryllium bromide BeBr2 magnesium oxide MgO magnesium oxide MgO **Omitted** H2O BI3 lithium sulfide Li2S aluminum sulfide Al2S3 **Omitted** BCl3 potassium oxide K2O aluminum oxide Al2O3 calcium fluoride CaF2 potassium sulfide K2S barium nitride Ba3N2 silver chloride AgCl cesium sulfide Cs2S calcium chloride CaCl2 strontium oxide SrO **Omitted** BN francium bromide FrBr cesium iodide CsI boron iodide Binary Ionic Compounds with Multivalent Cations: Recall that multivalent elements have multiple oxidation states (charges). When writing the names for compounds containing multivalent cations we must indicate the oxidation state of the cation. There are two methods for naming binary ionic compounds with multivalent cations. 1. The classical system (also referred to as the ous-ic method) 2. The IUPAC system (also referred to as the roman numeral method) The Classical System: 1. Use the Latin name of the cation in the chemical formula. Usually, the Latin name for Hg and Sb are not used. If the cation does not have a latin name, use the English name instead. 2. Remove the last syllable and add the suffix “-ous” or “-ic” in its place. a. The suffix “-ous” indicates the lower oxidation state was used for the cation. b. The suffix “-ic” indicates the higher oxidation state was used for the cation. (Note: arsenic’s name remains unchanged when the higher oxidation state is used.) 3. Write the name of the anion and change the ending to “-ide” as you have done previously. Classical and IUPAC Names of Common Multivalent Metal Ions Metal Ion Classical Name Fe2+ ferrous iron Fe3+ ferric Cu+ cuprous copper 2+ Cu cupric Sn2+ stannous tin Sn4+ stannic 2+ Pb plumbous lead Pb4+ plumbic 3+ Sb stibnous antimony Sb5+ stibnic 2+ Co cobaltous cobalt Co3+ cobaltic Au+ aurous gold 2+ Au auric Hg+ mercurous mercury 2+ Hg mercuric IUPAC Name iron(II) iron(III) copper(I) copper(II) tin(II) tin(IV) lead(II) lead(IV) antimony(III) antimony(V) cobalt(II) cobalt(III) gold(I) gold(II) mercury(I) mercury(II) Complete the following table for binary ionic compounds containing multivalent cations using the classical system. Chemical Name Chemical Formula Chemical Name auric iodide AuI2 cupric sulfide Chemical Formula CuS aurous sulfide Au2S cuprous sulfide Cu2S antimonic oxide Sb2O5 mercurous bromide HgBr antimonous chloride SbCl3 ferric oxide Fe2O3 mercuric oxide HgO ferrous oxide FeO mercurous fluoride HgF stannous fluoride SnF2 plumbous arsenide Pb3As2 stannic fluoride SnF4 plumbic nitride Pb3N4 **Omitted** MnBr7 stannic oxide SnO2 **Omitted** MnO stannous fluoride SnF2 plumbous chloride PbCl2 ferric sulfide Fe2S3 plumbic chloride PbCl4 ferrous hydride FeH2 antimonic sulfide Sb2S5 nickelic oxide Ni2O3 antimonous arsenide SbAs nickelous sulfide NiS **Omitted** AsI3 cuprous carbide Cu4C nickelous oxide NiO cupric oxide CuO cobaltous nitride CoN manganous phosphide **Omitted** ferrous fluoride FeF2 manganic chloride **Omitted** mercurous fluoride HgF2 Hg3As cupric chloride CuCl2 cobaltic iodide CoI3 stannic arsenide Sn3As4 arsenous oxide **Omitted** nickelic phosphide NiP Sb3N5 nickelous sulfide NiS arsenic oxide **Omitted** ferrous sulfide FeS antimonic nitride Sb3N5 (repeat) plumbic carbide PbC arsenic nitride **Omitted** mercurous oxide Hg2O cobaltous sulfide CoS cupric sulfide CuS plumbic oxide PbO2 cuprous sulfide Cu2S mercurous arsenide antimonic nitride The IUPAC System: 1. Write the English name of the multivalent metal cation first. 2. A Roman numeral indicating the positive charge on the cation is written in brackets after the cations name. NO SPACE is left between the cation name and the Roman numeral in brackets. 3. Write the name of the anion and change the ending to “-ide” as you have done previously. Complete the following table for binary ionic compounds containing multivalent cations using the IUPAC system. Chemical Name Chemical Formula Chemical Name Chemical Formula phosphorous(III) sulfide P2S3 copper(I) bromide CuBr phosphorus(V) oxide P2O5 copper(I) oxide Cu2O antimony(V) chloride SbCl5 mercury(I) chloride HgCl antimony(III) oxide Sb2O3 iron(III) oxide Fe2O3 mercury(II) fluoride HgF2 iron(II) sulfide FeS mercury(I) arsenide Hg3As tin(II) bromide SnBr2 lead(II) nitride Pb3N2 tin(IV) fluoride SnF4 lead(IV) oxide PbO2 manganese(IV) oxide MnO2 tin(II) fluoride SnF2 manganese(II) fluoride MnF2 tin(IV) sulfide SnS2 lead(II) iodide PbI2 iron(III) hydride FeH3 lead(IV) chloride PbCl4 iron(II) oxide FeO antimony(V) oxide Sb2O5 nickel(III) sulfide Ni2S3 antimony(III) arsenide SbAs nickel(II) carbide Ni2C **Omitted** AsF5 copper(I) oxide Cu2O **Omitted** N2O5 copper(II) phosphide Cu3P2 cobalt(III) arsenide CoAs manganese(II) chloride MnCl2 **Omitted** PBr5 manganese(VII) arsenide Mn3As7 **Omitted** PF3 carbon(II) iodide **Omitted** **Omitted** SF4 carbon(IV) oxide **Omitted** **Omitted** SAs2 arsenic(III) nitride **Omitted** nickel(III) phosphide NiP sulfur(IV) chloride **Omitted** nickel(II) oxide NiO arsenic(V) sulfide **Omitted** iron(II) sulfide FeS CoO lead(IV) carbide PbC **Omitted** mercury(I) sulfide Hg2S cobalt(II) oxide sulfur(VI) phosphide Ionic Compounds Containing Polyatomic Ions: Recall that polyatomic ions are groups of atoms that act as a single ion. Ionic compounds containing polyatomic ions are still comprised of a cation and an anion. However, the cation, or anion, may be a polyatomic ion. Fortunately there is only 1 polyatomic cation which we must remember – ammonium (NH4+) All other polyatomic ions have negative charges (are anions). Oxyanions: Polyatomic ions containing oxygen are called oxyanions. The number of oxygen atoms in an oxyanion can change without affecting the overall charge of the ion. Oxyanions follow a specific naming structure related to the number of oxygen atoms. The most stable and most common form of an oxyanion is given the suffix “-ate”. Oxyanions containing one or more oxygen atom than the “–ate” form of the ion are given the prefix “per-“ and the suffix “-ate”. Oxyanions containing one less oxygen atom than the “-ate” form of the ion use the suffix “-ite”. Oxyanions containing one less oxygen atom than the “-ite” form of the ion are given the prefix “hypo-“and the suffix “ite”. For Example: ClOClO2ClO3ClO4- hypochlorite chlorite chlorate perchlorate - has 1 less oxygen atom than the “-ite” form has 1 less oxygen atom than the “-ate” form. the most common and stable form of this oxyanion. has 1 more oxygen atom than the “-ate” form If you can remember the “-ate” form of the oxyanion you can use this relationship to determine the other chemical formula. In addition to oxyanions, there are a variety of polyatomic ions derived from acids and others which are considered miscellaneous. We will discuss the polyatomic ions derived from acids during our lesson on Acid/Base Nomenclature. Miscellaneous polyatomic ions can be looked up in your table of polyatomic ions. Steps for Naming Ionic Compounds Containing Polyatomic Ions: 1. Write the name of the cation. If it is a metal write the name. For multivalent elements be sure to include the charge. If it is ammonium just write ammonium. 2. Write the name of the polyatomic anion. Complete the following table of ionic compounds containing polyatomic ions using the IUPAC system. Chemical Name iron(II) sulfite Chemical Formula Chemical Name FeSO3 sodium sulfate Chemical Formula Na2SO4 nickel(III) carbonate Ni2(CO3)3 iron(II) hypophosphite Fe3(PO2)2 mercury(I) phosphite Hg3PO3 potassium chlorite KClO2 calcium perchlorate Ca(ClO4)2 silver hypobromite AgBrO radium nitrite Ra(NO2)2 sodium hypochlorite NaClO CoSO4 zinc carbonate ZnCO3 manganese(IV) periodate Mn(IO4)4 barium nitrite Ba(NO2)2 iron(II) hyponitrite Fe(NO)2 lithium sulfite Li2SO3 lithium bromate LiBrO3 magnesium perphosphate Mg3(PO5)2 cesium iodite CsIO2 lead(II) carbonate PbCO3 magnesium percarbonate MgCO4 calcium chlorate Ca(ClO3)2 barium hypophosphite BaPO2 iron(III) phosphate FePO4 manganese(II) nitrite Mn(NO2)2 tin(IV) sulfate Sn(SO4)2 tin(IV) sulfite Sn(SO3)2 aluminum hypophosphite AlPO2 magnesium periodate Mg(IO4)2 lead(II) iodate Pb(IO3)2 aluminum hyposulfite Al2(SO2)3 copper(I) phosphite Cu3PO3 lead(IV) perphosphate Pb3(PO5)4 mercury(II) sulfate HgSO4 tin(IV) sulfate Sn(SO4)2 antimony(V) sulfate Sb2(SO4)5 antimony(III) hyponitrite Sb(NO)3 calcium carbonate CaCO3 copper(II) chlorate Cu(ClO3)2 iron(II) carbonite FeCO2 cobalt(II) sulfate aluminum phosphite AlPO3 cesium perphosphate Cs3PO5 antimony(III) persulfate Sb2(SO5)3 cobalt(II) hyposulfite CoSO2 barium hyponitrite Ba(NO)2 copper(I) pernitrate CuNO4 beryllium carbonate BeCO3 iron(II) chlorite Fe(CIO2)2 Ca(ClO2)2 zinc iodate Zn(IO3)2 calcium chlorite Hydrates of Ionic Compounds: Many ionic compounds form crystal structures that contain molecules of water. These compounds are known as Hydrates. The water stored within the hydrate is known as Water of Hydration. Water of hydration can be removed from an associated ionic compound by heating. When the water is removed the ionic compound is referred to as Anhydrous. Writing Formula for Hydrates: The chemical formula for hydrates indicates the number of associated water molecules after the molecule. For Example: Hg(NO3)2 · H2O - this mercury(II) nitrate is associated with one water molecule. Naming Hydrates: 1. Name the ionic compound 2. Add the word “hydrate” with a Greek prefix (mono-, di-, tri-, etc.) indicating the number of water molecules associated with the ionic compound. For example: CoCl2 · 6 H2O is named cobalt(II) chloride hexahydrate. Complete the following table of Hydrates using the IUPAC system. Chemical Name manganese(II) chromate pentahydrate Chemical Formula Chemical Name Chemical Formula MnCrO4 · 5 H2O calcium sulfate dehydrate CaSO4 · 2 H2O Mg(NO3)2 · 6 H2O iron(III) chloride hexahydrate FeCl3 · 6 H2O SnCl2 · 2 H2O barium hydroxide octahydrate Ba(OH)2 · 8 H2O mercury(I) nitrate dihydrate HgNO3 · 2 H2O chromium(III) bromide hexahydrate CrBr3 · 6 H2O lead(II) perchlorate trihydrate Pb(ClO4)2 · 3 H2O copper(II) perchlorate hexahydrate Cu(ClO4)2 · 6 H2O copper(II) acetate monohydrate Cu(C2H3O2)2 · H2O magnesium sulfate heptahydrate MgSO4 · 7 H2O manganese(IV) periodate Mn(IO4)4 (oops forgot the water!) iron(II) fluoride tetrahydrate FeF2 · 4 H2O barium chloride dihydrate BaCl2 · 2 H2O copper(II) chloride dihydrate CuCl2 · 2 H2O magnesium nitrate hexahydrate tin(II) chloride dihydrate









