Gay-Lussac's Law and Temperature
advertisement
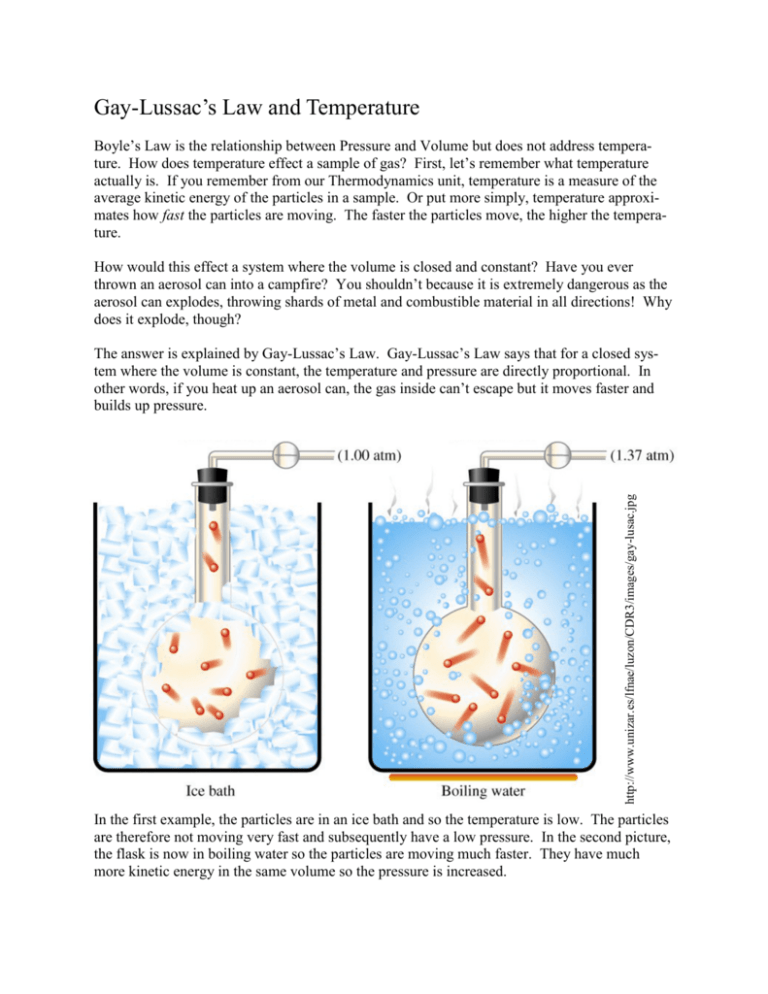
Gay-Lussac’s Law and Temperature Boyle’s Law is the relationship between Pressure and Volume but does not address temperature. How does temperature effect a sample of gas? First, let’s remember what temperature actually is. If you remember from our Thermodynamics unit, temperature is a measure of the average kinetic energy of the particles in a sample. Or put more simply, temperature approximates how fast the particles are moving. The faster the particles move, the higher the temperature. How would this effect a system where the volume is closed and constant? Have you ever thrown an aerosol can into a campfire? You shouldn’t because it is extremely dangerous as the aerosol can explodes, throwing shards of metal and combustible material in all directions! Why does it explode, though? http://www.unizar.es/lfnae/luzon/CDR3/images/gay-lusac.jpg The answer is explained by Gay-Lussac’s Law. Gay-Lussac’s Law says that for a closed system where the volume is constant, the temperature and pressure are directly proportional. In other words, if you heat up an aerosol can, the gas inside can’t escape but it moves faster and builds up pressure. In the first example, the particles are in an ice bath and so the temperature is low. The particles are therefore not moving very fast and subsequently have a low pressure. In the second picture, the flask is now in boiling water so the particles are moving much faster. They have much more kinetic energy in the same volume so the pressure is increased. Experiment In this experiment we will be graphing Gay-Lussac’s Law. An empty beaker of air is sealed with a stopper and then attached to a pressure sensor. The beaker will then be put in boiling water and then in an ice bath. Pressure readings will be taken at each of the three temperatures. http://web.njit.edu/~grow/sensors/Pprobe_files/image005.gif Pressure (mm Hg) Temperature (oC) 703 0 760 22 961 100 Pressure vs Tem perature 1200 Pressure (mm Hg) 1000 800 600 400 200 0 0 20 40 60 Tem perature (Celcius) 80 100 120 Results We can see from the graph that there is a direct relationship between the temperature and the pressure. As the temperature went up, so did the pressure. As the temperature went down, so did the pressure. Using the same idea as before in Boyle’s Law: y = mx + b P = mT + b In the Boyle’s Law graph, it was obvious that the y-intercept, b, was zero. But look again at this graph, when the temperature is zero, there is still pressure. Note that the line doesn’t cross at 0,0 Even if we ignore that, we have another problem. The ratio of P/T should be constant amount P=m T But if we do the math, it doesn’t work out: Temperature (Celcius) Pressure (mm Hg) P/T 100 961 9.61 22 760 34.5 0 703 ??? We need to fix these problems. But to do so is going to require rectifying the math with the theory. Rectification The first thing we have to address is the theory behind this relationship. We are saying that the hotter the particles get, the faster they move in a closed container, and thus the more pressure they exert. What if we took that in the other direction? What happens to the particles as they get colder? They should move slower. Ultimately, the coldest they can get is if there is no molecular motion at all. What should the pressure be at that point? If there is no molecular motion, there should be no pressure. The coldest a particle can get is if there is no motion at all. What is this temperature? We can estimate it by using our graph from before: We can see that by this graph, there is still pressure at 0 oC. Particles must be able to get colder than this. How cold? As said above, if the pressure is 0, there should be no molecular motion and therefore no temperature. So if we extend out the graph until the line touches 0 mm Hg, that should approximately be the coldest possible temperature: Pressure vs. Tem perature 1200 1000 y = 2.5792x + 703.11 Pressure (mm Hg) 800 600 Pressure is zero here so this is the coldest possible temperature 400 200 0 -300 -250 -200 -150 -100 -50 Tem perature (Celcius) 0 50 100 Absolute (Kelvin) Temperature The graph on the previous page leads us to an important distinction about temperature. If we remember, the two temperature scales we are used to, Fahrenheit and Celcius, were arbitrarily picked by their creators. Daniel Fahrenheit picked 32 oF as the point that water freezes and 212 o F as the point that water boiled. Celcius picked 0 oC as the point that water freezes and 100 oC as the point that water boils. They are both temperature scales that allow negative numbers. To fix this, William Thomson, aka Lord Kelvin, devised a temperature scale that makes sense. In his scale, the lowest possible temperature should be zero. This is the absolute lowest temperature that could be reached. He called this temperature absolute zero and it is the temperature where there is no molecular motion at all. This temperature, if you look at the graph on the previous page is –273 oC. So to make sure there are no negative numbers, the Kelvin scale simply adds 273 to any Celcius temperature. o C + 273 = K You’ll notice that there is no o symbol by the Kelvin in the above equation. Because Kelvin is the absolute scale and not a relative scale like Celcius and Fahrenheit, it doesn’t need the o symbol! http://www.lyc-schweitzermulhouse.ac-strasbourg.fr/spip/IMG/ jpg/image004-4.jpg http:// www.biografiasyvidas.com/ biografia/c/fotos/celsius.jpg http://sol.sci.uop.edu/ ~jfalward/ temperatureandexpansion/ LordKelvin.jpg Daniel Fahrenheit lived from 1686 to 1736. He was German by birth but lived most of his life in the Dutch Republic. In 1724 he proposed his scale that water boils at 32 oF and freezes at 212 oF. It was later reversed for obvious reasons. Why the temperatures of 32 and 212 were picked is not known and is the subject of much debate. The Fahrenheit scale is used only in the US and a few other countries. Anders Celcius lived from 1701 to 1744 in Sweden. In 1742 he proposed a scale where water boils at 0 oC and freezes at 100 oC. One year later it, like the Fahrenheit scale, was reversed to the scale we now know. The Celcius scale is used throughout most of the world. William Thomson, aka Lord Kelvin, lived from 1824 to 1907 in Belfast, Ireland. His Kelvin temperature scale is one of many different contributions to the field of Thermodynamics and other aspects of Science and Engineering. Putting it All Together We now need to combine all of the information from the previous sections. Let’s quick review: Gay-Lussac’s Law: Pressure is directly related to Temperature for a system of constant volume Temperature is a measure of average kinetic energy Temperature can be measured in an absolute scale by adding 273 to any Celcius temperature Now let’s examine the relationship from before but this time use Kelvin Temperature P = mT + b But since b is now zero: P=m T Pressure (mm Hg) Temperature (Kelvin) P/T 961 373 2.58 760 295 2.58 703 273 2.58 This one works perfectly! As long as there are no negative numbers allowed, the ratio in this relationship is right on target. You will notice that the slope of the line is the 2.58 that we predicted! Conclusion & Gay-Lussac’s Law The relationship between pressure and temperature is a direct one that works perfectly when Kelvin temperatures are used. We come to the conclusion: P=m T P = 2.58 T The fact that it is 2.58, though, shouldn’t even come up. Look at experiments #1 & 3: 961 = 2.58 373 703 = 2.58 273 Let’s cut out the middle-man: 961 = 703 373 273 Or to put another way: P1 = P2 T1 T 2 Temperature here MUST be converted to Kelvin for this to work! This is a statement of Gay-Lussac’s Law. The pressure of a gas at constant volume is directly related to its Kelvin temperature. Standard Temperature and Pressure (STP) With all these pressures and temperatures flying about, it was decided to have a standard temperature and pressure. To keep things easy, the standard was set at 0 oC (273 K) and a pressure of 1 atm (14.7 psi or 760 mm Hg or 760 torr or 101 kPa). Joseph Louis Gay-Lussac was a French chemist who lived from 1778 to 1850. Aside from the law that bears his name he was the co-discoverer of the element Boron and coined the terms ―pipette‖ and ―burette‖. http://creationwiki.org/pool/images/ thumb/2/2f/Gaylussac.jpg/150pxGaylussac.jpg Gay-Lussac’s Law Questions: A) A sample of gas in a closed, rigid container at 750 mm Hg is at a temperature of 22 o C. What pressure would the gas exert at a temperature of 100 oC? P1 = 750 mm Hg T1 = 22 oC = 295 K P2 = X T2 = 100 oC = 373 K 750 mm Hg 295 K = X 373 K Cross multiply and divide: (750 mm Hg)(373 K) = (750 mm Hg)(373 K) = (295 K) X(295 K) X X = 948 mm Hg B) At what temperature will a sample of gas in a closed container reach a pressure of 5 atm if at standard temperature (0 oC) the gas is at 1 atm? P1 = 5 atm T1 = X P2 = 1 atm T2 = 0 oC = 273 K Be careful, the X is in the denominator here! 5 atm = X 1 atm 273 K Cross multiply and divide: (5 atm)(273 K) = (5 atm)(273 K) (1 atm) = X(1 atm) X X = 1365 K If you want it in oC then you must subtract 273 X = 1365—273 = 1092 oC Questions 1. 2. 3. 4. 5. Gay-Lussac’s Law is the relationship between what two variables? Which variable must be constant in Gay-Lussac’s Law? Why should you never throw a closed container like an aerosol can in a campfire? What is different about the Kelvin temperature scale compared to Celcius and Fahrenheit? For each temperature below convert either from Celcius to Kelvin or the reverse: a) 50 oC to K b) 110 oC to K c) -200 oC to K d) 0 C to K e) 400 K to oC f) 100 K to oC g) 1250 K to to oC 6. Sketch the curve of the graph of Pressure vs. Temperature: P T 7. What are standard temperature and pressure? 8. A sample of gas at 1.5 atm and 300 K is heated to 500 K. What is the new pressure? 9. A sample of gas at 750 mm Hg and 20 oC is cooled to –40 oC. What is the new pressure? 10. A sample of gas at 120 kPa and 25 oC is heated until the pressure is 200 kPa. What is the temperature (in both K and oC) that this occurs? 11. A sample of gas will explode out of its container if the pressure reaches 3 atm. What temperature (in both K and oC) will this occur if the can is currently at standard temperature and pressure?



![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)




