Siemens Healthcare Diagnostics Inc.
IMMULITE® 2000
IMMULITE® 2000 XPi
Customer Bulletin
CB2011-10-03
2011-10
IMMULITE 2000/IMMULITE 2000 XPi Androstenedione Precision
Improvement
Siemens Healthcare Diagnostics is announcing precision improvements to the
IMMULITE 2000/IMMULITE 2000 XPi Androstenedione (L2KAO2 – SMN #
10381188) assay.
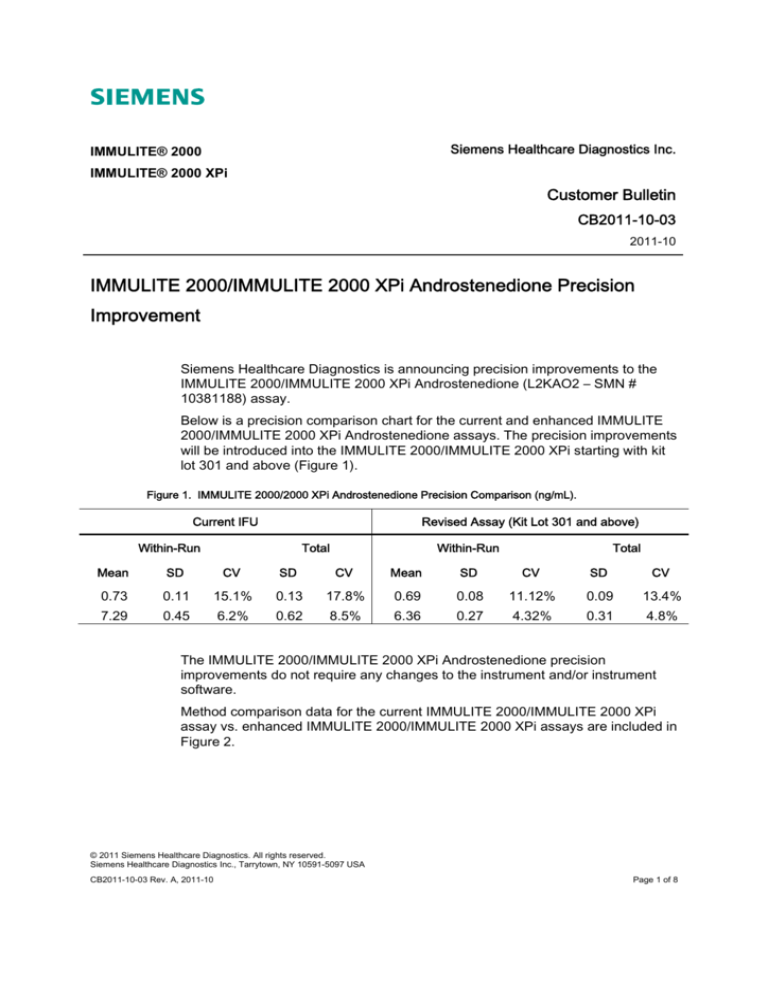
Below is a precision comparison chart for the current and enhanced IMMULITE
2000/IMMULITE 2000 XPi Androstenedione assays. The precision improvements
will be introduced into the IMMULITE 2000/IMMULITE 2000 XPi starting with kit
lot 301 and above (Figure 1).
Figure 1. IMMULITE 2000/2000 XPi Androstenedione Precision Comparison (ng/mL).
Current IFU
Revised Assay (Kit Lot 301 and above)
Within-Run
Total
Within-Run
Total
Mean
SD
CV
SD
CV
Mean
SD
CV
SD
CV
0.73
0.11
15.1%
0.13
17.8%
0.69
0.08
11.12%
0.09
13.4%
7.29
0.45
6.2%
0.62
8.5%
6.36
0.27
4.32%
0.31
4.8%
The IMMULITE 2000/IMMULITE 2000 XPi Androstenedione precision
improvements do not require any changes to the instrument and/or instrument
software.
Method comparison data for the current IMMULITE 2000/IMMULITE 2000 XPi
assay vs. enhanced IMMULITE 2000/IMMULITE 2000 XPi assays are included in
Figure 2.
© 2011 Siemens Healthcare Diagnostics. All rights reserved.
Siemens Healthcare Diagnostics Inc., Tarrytown, NY 10591-5097 USA
CB2011-10-03 Rev. A, 2011-10
Page 1 of 8
IMMULITE/1000/2000/2000XPi Androstenedione Precision Improvement
Figure 2. IMMULITE 2000/IMMULITE 2000 XPi Current vs. Enhanced Method Comparison.
Enhanced L2KAO
15
10
5
0
0
5
10
15
Current L2KAO
Enhanced L2KAO = 0.936 × Current L2KAO – 0.0331
CB2011-10-03 Rev. A, 2011-10
Page 2 of 8
IMMULITE/1000/2000/2000XPi Androstenedione Precision Improvement
A reference range study was performed on serum samples from 54 male and 57
female apparently normal, healthy adult laboratory volunteers using the
enhanced IMMULITE 2000/IMMULITE 2000 XPi Androstenedione assay.
Revised reference range guidelines for the IMMULITE 2000/IMMULITE 2000 XPi
enhanced assay are provided in Figure 3 below.
Figure 3. IMMULITE 2000/IMMULITE 2000 XPi Current and Revised Reference Range Comparison.
IMMULITE
Current IFU Reference Range
2000/
Reference Range from Enhanced Assay
Males
Females
Males
Females
1.6 ng/mL
1.7 ng/mL
(5.6 nmol/L)
(5.9 nmol/L)
1.1 ng/mL
(3.8nmol/L)
1.1 ng/mL
(3.8nmol/L)
Central 95%
0.6 – 3.1 ng/mL
0.3 – 3.3 ng/mL
0.4 – 2.6 ng/mL
0.4 – 4.1 ng/mL
Range
(2.1–10.8 nmol/L)
(1.0–11.5 nmol/L)
(1.4–9.1 nmol/L)
(1.4–14.3 nmol/L)
2000 XPi
Median
Be aware that reference range data is population dependent, and for this reason,
the reference ranges provided should be considered as guidelines only. Each
laboratory should establish its own reference ranges.
Control Information
The improvements made to the IMMULITE 2000/IMMULITE 2000 XPi
Androstenedione assays have led to a positive shift in CON6 Control values.
Revised target values are provided below.
Figure 4. CON6 lot 023 Revised Control Targets.
Platform
Control
IMMULITE 2000/2000
XPi
CB2011-10-03 Rev. A, 2011-10
Previous
Revised
Mean
Mean
SD
2SD Range
CON65023
3.4
4.0
0.40
3.20 – 4.80
CON66023
8.5
8.5
0.75
7.00 – 10.00
Page 3 of 8
IMMULITE/1000/2000/2000XPi Androstenedione Precision Improvement
There may be an increased incidence of the CON6 lot 023 level 6 control
material resulting above the assay’s reportable range with the enhanced assay.
The Bio-Rad Immunoassay Lyphochek® or Bio-Rad Immunoassay Liquichek™
control material can be used as an alternative. Figures 5 and 6 provide
experienced control targets for multiple Bio-Rad Immunoassay Lyphochek or BioRad Immunoassay Liquichek lots.
Figure 5. Bio-Rad Lyphochek experienced control targets (ng/mL).
Platform
IMMULITE 2000/
IMMULITE 2000 XPi
Control
Mean
SD
2SD Range
40231
1.15
0.19
0.77 – 1.53
40232
6.11
0.48
5.14 – 7.08
40241
1.30
0.21
0.88 – 1.72
40242
5.80
0.64
4.52 – 7.08
40251
1.40
0.22
0.95 – 1.85
40252
5.72
0.63
4.46 – 6.98
Figure 6. Bio-Rad Liquichek experienced control targets (ng/mL).
Platform
IMMULITE 2000/
IMMULITE 2000 XPi
CB2011-10-03 Rev. A, 2011-10
Control
Mean
SD
2SD Range
40751
0.96
0.15
0.65 – 1.27
40752
3.20
0.35
2.49 – 3.90
40753
6.86
0.75
5.35 – 8.36
40761
1.18
0.17
0.80 – 1.56
40762
3.32
0.37
2.59 – 4.05
40763
7.78
0.86
6.07 – 9.49
40771
1.07
0.17
0.73 – 1.41
40772
2.80
0.31
2.18 – 3.42
40773
8.69
0.96
6.78 – 10.00
40781
1.15
0.18
0.78 – 1.52
40782
3.17
0.35
2.47 – 3.87
40783
8.47
0.93
6.61 – 10.00
Page 4 of 8
IMMULITE/1000/2000/2000XPi Androstenedione Precision Improvement
Important Notices
Important Notices as shown below will be packed with L2KAO kit lots 301 and
above.
IMPORTANT NOTICE
IMMULITE 2000/IMMULITE 2000 XPi
Androstenedione
Kit lots affected:
Lot 301 and above
2011-07-29 Refer to table below for revised targets and ranges in ng/mL.
Previous
Revised
Level
Mean
Mean
SD
2SD Range
CON65023
3.4
4.0
0.40
3.2 – 4.8
IMPORTANT NOTICE
IMMULITE® 2000/IMMULITE 2000 XPi
Androstenedione
Kit lots affected:
Lot 301 and above
The precision of the assay has been improved. A
reference range study was performed using the
enhanced assay on serum samples from 54 males
and 57 female adult laboratory volunteers using the
IMMULITE 2000 Androstenedione procedure.
Males: Median: 1.1ng/mL (3.8nmol/L).
Central 95% range: 0.4-2.6ng/mL (1.4-9.1nmol/L).
Females: Median: 1.1ng/mL (3.8nmol/L).
Central 95% range: 0.4-4.1ng/mL (1.4-14.3nmol/L).
Consider these limits as guidelines only. Each
laboratory should establish its own reference
ranges.
CB2011-10-03 Rev. A, 2011-10
Page 5 of 8
IMMULITE/1000/2000/2000XPi Androstenedione Precision Improvement
IFU Changes
The IMMULITE 2000/IMMULITE 2000 XPi Androstenedione IFU will be updated.
IFU updates will include revisions of the Precision Tables and Expected Values
sections to better reflect enhanced assay performance.
Thank you for your attention to this customer bulletin. We appreciate your
continued business.
Availability and Ordering Information
The enhanced L2KAO kit lot 301 and above will be available for shipping on
October 10, 2011.
Kits with current precision performance will ship routinely until December 12,
2011. To obtain enhanced performance kits before December 12, specify kit lot
number when ordering. After December 12, 2011, only the improved precision
kit lots will ship routinely; older kits will be available upon request while stock is
available. Customers should begin to use the improved precision kits as soon as
possible. Availability is summarized below (Figure 7).
Figure 7. Current and Enhanced Precision Performance Kit Availability.
Kit
Up to December 12, 2011
After December 12, 2011
Current (L2KAO kit lot 300 and below)
Will ship routinely
Specify kit lot number
when ordering
Enhanced Precision (L2KAO kit lot
301 and above)
Specify kit lot number
when ordering
Will ship routinely
CB2011-10-03 Rev. A, 2011-10
Page 6 of 8
IMMULITE/1000/2000/2000XPi Androstenedione Precision Improvement
Frequently Asked Questions
Questions
Will there be changes in Quality
Control Material target values?
Answers
Yes. CON6 lot 023 target revision information is
provided within this bulletin. There may be an
increased incidence of the CON6 lot 023 level 6
control material resulting above the assay’s
reportable range. The Bio-Rad Immunoassay
Lyphochek or Bio-Rad Immunoassay Liquichek
control material can be used as an alternative.
Expected ranges for the Bio-Rad Immunoassay
Lyphochek and Bio-Rad Immunoassay Liquichek
are also included in this bulletin.
Why are Bio-Rad control values within
this bulletin labeled Experienced
Ranges?
Siemens does not routinely run Bio-Rad controls inhouse. Therefore, only experienced ranges are
provided.
These experienced ranges have been forwarded to
Bio-Rad for inclusion in applicable IFUs at BioRad’s discretion.
Is it necessary to carry out method
comparison studies between current
and enhanced kit lots?
Siemens performed method comparison studies
during the development of the enhanced assay –
method comparison charts are included in this
bulletin.
If method comparisons are desired, both current
and enhanced precision kits will be available for
approximately 6 months. Current kits will be
shipped routinely until December 12, 2011. The
improved precision kit lots will be available upon
request on October 10, 2011 – please state kit lot
L2KAO kit lot 301 and above when placing an order.
Why is Siemens improving the
IMMULITE 2000/IMMULITE 2000 XPi
Androstenedione precision
performance?
The IMMULITE 2000/IMMULITE 2000 XPi
Androstenedione IFU was updated in 2010 to reflect
an observed increase in assay imprecision.
Siemens is now restoring the assay to its historical
precision performance.
Will there be any changes to the
IMMULITE 2000/IMMULITE 2000 XPi
Androstenedione IFUs?
Yes. IFU precision table and reference range data
will be updated to better reflect enhanced assay
performance.
Will normal reference ranges need to
be reset?
Yes. Reference ranges will need to be reset based
on the guidelines provided in this bulletin and
included in an Important Notice to be packed with
the enhanced precision kits.
CB2011-10-03 Rev. A, 2011-10
Page 7 of 8
IMMULITE/1000/2000/2000XPi Androstenedione Precision Improvement
Trademark Information
IMMULITE ® is a trademark of Siemens Healthcare Diagnostics.
Lyphochek® and Liquichek™ are trademarks of Bio-Rad laboratories, Inc.
Additional Assistance
If you have any questions or need additional information, please contact your
local service organization.
CB2011-10-03 Rev. A, 2011-10
Page 8 of 8